Press release
CloudLIMS Accelerates COVID-19 Testing & Research with its Preconfigured, Rapid Deployment COVID-19 LIMS
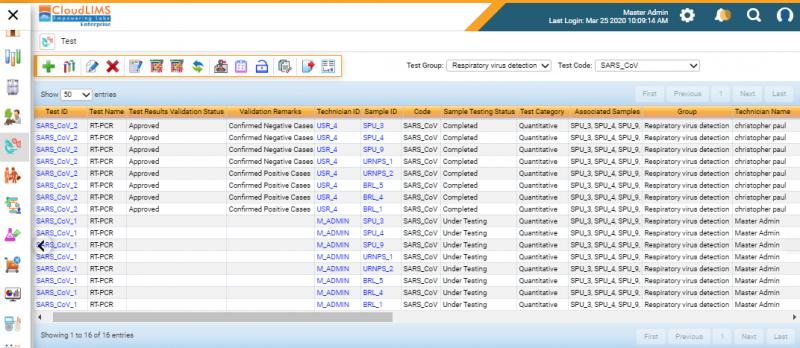
Wilmington, Delaware - April 30, 2020, CloudLIMS, a leading laboratory informatics provider, announces the release of a rapid deployment LIMS, preconfigured for COVID-19 testing. The LIMS can be quickly set up for COVID-19 testing, in 2 to 4 days, comes pre-loaded with FDA and CDC approved tests, report templates, and workflows to maximize testing and reporting capability of COVID-19 laboratories. This functionality enables COVID-19 diagnostic testing laboratories to manage a massive influx of COVID-19 specimens and to quickly turnaround test specimens and report results as per FDA and CDC guidelines.
Combined with the previous announcement of offering its flagship product, CloudLIMS Enterprise, free of charge to COVID-19 research and testing laboratories across the globe, CloudLIMS now extends support to such laboratories by offering its rapid deployment, preconfigured product. Read the previous press release here.
The demand for COVID-19 testing is growing exponentially with over 3 million confirmed cases globally. CloudLIMS, an in the cloud COVID-19 LIMS, is preconfigured with COVID-19 specific tests, such as RT-PCR, serological tests, workflows, and reporting templates, enabling laboratories to go-live in less than a week. CloudLIMS can be seamlessly configured to support new and upcoming test methods, assays, and reporting requirements, for COVID-19 and other infectious diseases, avoiding costly and time-consuming customization. CloudLIMS' client portal enables physicians to place SARS-CoV-2 diagnostic test requests, track request status in real-time, download test reports, minimizing the turnaround time.
CloudLIMS' REST API is preconfigured to support integration with COVID-19 testing instruments, such as RT-PCR, NGS instruments. Furthermore, the Rest API supports integration with third-party software such as EMR, EHR, LIS, patient registries, and clinical billing software. CloudLIMS seamlessly integrates with the reporting system of CDC/CSTE/FEMA and other e-reporting tools to automate the electronic reporting of results.
CloudLIMS enables laboratories to securely manage COVID-19 specimens, anonymize patient data, tests and test results, ensure quality control, schedule instrument calibration, manage training and competency of laboratory staff handling biohazardous specimens. CloudLIMS' COVID-19 LIMS also enables clinical laboratories to follow regulatory guidelines and standards such as CLIA, HIPAA, ISO 15189:2012.
"Our team has further developed and preconfigured CloudLIMS to meet the urgent requirements of testing and research of COVID-19 in these unprecedented and challenging times," said Arun Apte, Chief Executive Officer at CloudLIMS. "CloudLIMS can be deployed in less than a week and has the potential to maximize the testing and reporting capability of nascent and existing COVID-19 testing laboratories while maintaining FDA, CDC, and other regulatory guidelines. We hope to empower a greater number of laboratories to accelerate COVID-19 testing and research and help the world overcome the global health crisis," he continued.
Contact:
Ankita Acharya
CloudLIMS.com
302-789-0447
support@cloudlims.com
About CloudLIMS
Established in 2013, CloudLIMS is an energetic team of professionals producing cutting edge laboratory software solutions, such as sample management software and LIMS. For more information, please visit: www.cloudlims.com.
Combined with the previous announcement of offering its flagship product, CloudLIMS Enterprise, free of charge to COVID-19 research and testing laboratories across the globe, CloudLIMS now extends support to such laboratories by offering its rapid deployment, preconfigured product. Read the previous press release here.
The demand for COVID-19 testing is growing exponentially with over 3 million confirmed cases globally. CloudLIMS, an in the cloud COVID-19 LIMS, is preconfigured with COVID-19 specific tests, such as RT-PCR, serological tests, workflows, and reporting templates, enabling laboratories to go-live in less than a week. CloudLIMS can be seamlessly configured to support new and upcoming test methods, assays, and reporting requirements, for COVID-19 and other infectious diseases, avoiding costly and time-consuming customization. CloudLIMS' client portal enables physicians to place SARS-CoV-2 diagnostic test requests, track request status in real-time, download test reports, minimizing the turnaround time.
CloudLIMS' REST API is preconfigured to support integration with COVID-19 testing instruments, such as RT-PCR, NGS instruments. Furthermore, the Rest API supports integration with third-party software such as EMR, EHR, LIS, patient registries, and clinical billing software. CloudLIMS seamlessly integrates with the reporting system of CDC/CSTE/FEMA and other e-reporting tools to automate the electronic reporting of results.
CloudLIMS enables laboratories to securely manage COVID-19 specimens, anonymize patient data, tests and test results, ensure quality control, schedule instrument calibration, manage training and competency of laboratory staff handling biohazardous specimens. CloudLIMS' COVID-19 LIMS also enables clinical laboratories to follow regulatory guidelines and standards such as CLIA, HIPAA, ISO 15189:2012.
"Our team has further developed and preconfigured CloudLIMS to meet the urgent requirements of testing and research of COVID-19 in these unprecedented and challenging times," said Arun Apte, Chief Executive Officer at CloudLIMS. "CloudLIMS can be deployed in less than a week and has the potential to maximize the testing and reporting capability of nascent and existing COVID-19 testing laboratories while maintaining FDA, CDC, and other regulatory guidelines. We hope to empower a greater number of laboratories to accelerate COVID-19 testing and research and help the world overcome the global health crisis," he continued.
Contact:
Ankita Acharya
CloudLIMS.com
302-789-0447
support@cloudlims.com
About CloudLIMS
Established in 2013, CloudLIMS is an energetic team of professionals producing cutting edge laboratory software solutions, such as sample management software and LIMS. For more information, please visit: www.cloudlims.com.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
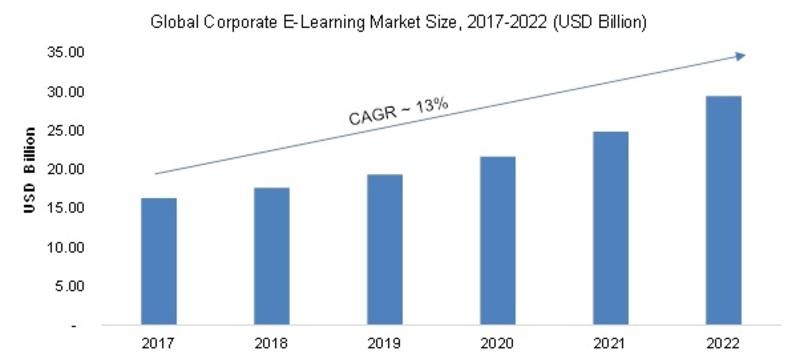
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...