Press release
Electronic Trial Master File (eTMF) Systems Market: A Detailed Overview
Introduction
The Electronic Trial Master File (eTMF) systems market has become a vital segment in the clinical research industry. eTMF systems are digital solutions designed to manage the documentation and compliance requirements for clinical trials. These systems allow pharmaceutical, biotechnology, and medical device companies to store, manage, and track trial master file documents electronically, which is essential for ensuring regulatory compliance, improving operational efficiency, and enhancing data accessibility during clinical trials. The need for eTMF systems has grown as clinical trials become more complex, and the demand for faster and more efficient document management solutions has increased. These systems play a key role in facilitating smooth, efficient trial processes, supporting collaboration, and ensuring data integrity throughout a trial's lifecycle.
For more information:
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Market Size
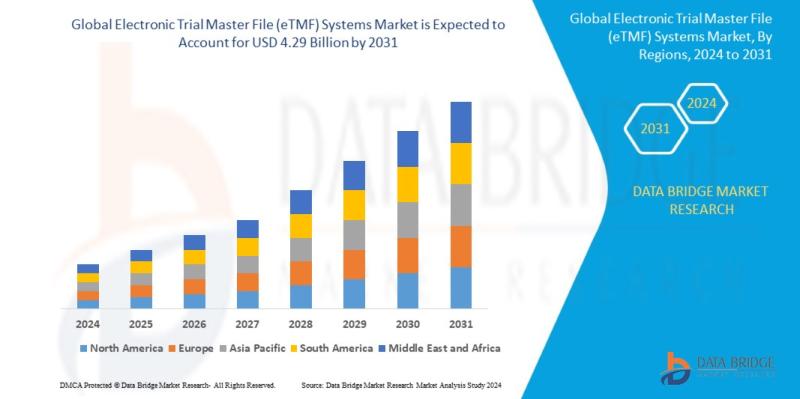
Global electronic trial master file (ETMF) systems market size was valued at USD 1.63 billion in 2023 and is projected to reach USD 4.29 billion by 2031, with a CAGR of 12.9% during the forecast period of 2024 to 2031. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand.
Market Share
North America dominates the global eTMF systems market, accounting for a significant portion of the total market share. The United States, in particular, leads due to its advanced healthcare infrastructure, a large number of clinical trials, and a high adoption rate of digital technologies. The presence of key market players in North America, including software providers and regulatory bodies like the U.S. Food and Drug Administration (FDA), further strengthens the region's dominance. Additionally, stringent regulations regarding clinical trial documentation in North America drive the demand for eTMF systems in the region.
Europe follows closely, with countries such as the UK, Germany, and France adopting eTMF systems in both clinical trial management and regulatory compliance. The European Medicines Agency (EMA) has also supported the adoption of electronic systems for clinical trials, which has led to increased adoption in the region.
The Asia-Pacific region is experiencing significant growth in the eTMF systems market due to the rise in clinical trials, particularly in countries like China and India. The rapid development of healthcare infrastructure, increased outsourcing of clinical trials to Asia, and growing pharmaceutical and biotechnology industries are contributing factors to this market expansion. Although the eTMF market is still emerging in these regions, the increasing focus on regulatory compliance and technological advancements are likely to drive future growth.
Market Opportunities and Challenges
Opportunities
Rising Number of Clinical Trials: As the demand for new treatments and medications continues to grow, the number of clinical trials has increased significantly. This surge presents an opportunity for eTMF systems, as they are essential for managing the vast amount of documentation associated with clinical trials. As pharmaceutical and biotechnology companies expand their clinical trial activities, the demand for efficient and secure document management systems is expected to rise.
Regulatory Requirements for Electronic Documentation: Regulatory agencies around the world are increasingly mandating electronic systems for managing clinical trial documentation. In particular, the FDA and EMA have adopted regulations requiring electronic systems for trial documentation management, further driving the demand for eTMF solutions. The growing need for compliance with Good Clinical Practice (GCP) guidelines and regulatory standards is creating significant opportunities for eTMF providers to offer solutions that streamline document management.
Cloud-Based Solutions and AI Integration: The shift toward cloud-based eTMF solutions offers several advantages, including reduced infrastructure costs, easier access to data, and the ability to scale operations. The integration of Artificial Intelligence (AI) and machine learning into eTMF systems is an exciting opportunity. These technologies can improve document categorization, automate processes, and enhance data security, which are highly valuable in the management of clinical trial documentation.
Outsourcing of Clinical Trials: The growing trend of outsourcing clinical trials to Contract Research Organizations (CROs) and third-party providers is driving the demand for centralized eTMF systems that can be accessed remotely. These solutions provide a collaborative platform for sponsors, CROs, and other stakeholders to access trial documents in real time, facilitating smoother trial operations and improving efficiency.
Challenges
High Implementation Costs: One of the main challenges in the eTMF systems market is the high cost of implementing and maintaining these systems, especially for small and mid-sized pharmaceutical companies. The cost of software, training, and support services can be prohibitive, which may limit the adoption of eTMF systems in certain markets.
Data Security and Privacy Concerns: With the increasing reliance on electronic systems, ensuring data security and compliance with privacy regulations becomes a challenge. Clinical trial data is highly sensitive, and breaches can lead to regulatory penalties and damage to a company's reputation. Addressing these security concerns through advanced encryption and secure data storage is critical for eTMF providers.
Integration with Legacy Systems: Many organizations are still using legacy systems to manage their clinical trial documentation. Integrating eTMF systems with these outdated systems can be a complex and time-consuming process, which may hinder the transition to more efficient electronic systems. Compatibility issues between different software solutions can also pose challenges for organizations looking to modernize their document management processes.
User Adoption and Training: The successful implementation of eTMF systems requires adequate training and support for users. Resistance to change and a lack of familiarity with digital systems can slow down adoption, especially among clinical trial teams that have been accustomed to traditional paper-based document management methods. Overcoming these barriers requires comprehensive training programs and user-friendly systems that streamline the transition to electronic documentation.
Market Demand
The demand for eTMF systems is driven by the increasing complexity of clinical trials and the need for efficient, secure document management solutions. As clinical trials grow in scope and scale, the volume of data and documentation generated has skyrocketed. Traditional paper-based document management methods are no longer sufficient to handle the volume of data generated by modern clinical trials. eTMF systems address this need by offering secure, organized, and easily accessible digital storage solutions.
Additionally, the need for compliance with regulatory standards such as GxP (Good Clinical Practice) and 21 CFR Part 11 has fueled the demand for eTMF systems. These systems provide a secure, compliant framework for managing clinical trial documents, reducing the risk of regulatory non-compliance.
As the number of clinical trials increases and more companies move towards digital solutions, the demand for eTMF systems is expected to grow steadily. The ability to centralize documents and provide real-time access to authorized stakeholders, such as sponsors, regulatory bodies, and research teams, makes eTMF systems an invaluable tool in modern clinical trial management.
Market Trends
Cloud Adoption: Cloud-based eTMF solutions are rapidly gaining popularity due to their scalability, flexibility, and cost-effectiveness. Cloud technology allows companies to store vast amounts of trial data in a secure, centralized location, making it accessible from anywhere at any time. The cloud-based approach also reduces the burden of maintaining on-premise infrastructure, lowering operational costs.
Automation and AI Integration: eTMF systems are increasingly incorporating automation and AI to improve efficiency. Automated workflows and AI-powered document categorization help streamline the document management process, reducing manual intervention and minimizing the risk of errors. These advancements also enhance compliance by ensuring that trial documents are organized, tagged, and indexed correctly.
Collaborative Platforms: There is a growing trend toward integrating eTMF systems with other clinical trial management tools, creating a collaborative platform where stakeholders can share and manage trial data seamlessly. This integration helps improve communication, reduces duplication of effort, and accelerates the clinical trial process.
Focus on Compliance and Security: As regulatory requirements become more stringent, there is an increasing focus on ensuring that eTMF systems are fully compliant with industry standards and regulations. Security features such as end-to-end encryption, secure user authentication, and audit trails are being integrated into eTMF systems to ensure that data is protected throughout the trial lifecycle.
Browse Trending Reports:
https://newsresearch12.blogspot.com/2024/12/balo-disease-treatment-market-size.html
https://newsresearch12.blogspot.com/2024/12/high-temperature-insulation-materials.html
https://newsresearch12.blogspot.com/2024/12/cytokinin-market-size-share-trends_10.html
https://newsresearch12.blogspot.com/2024/12/flowers-and-ornamental-plants-market.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com"
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
The Electronic Trial Master File (eTMF) systems market has become a vital segment in the clinical research industry. eTMF systems are digital solutions designed to manage the documentation and compliance requirements for clinical trials. These systems allow pharmaceutical, biotechnology, and medical device companies to store, manage, and track trial master file documents electronically, which is essential for ensuring regulatory compliance, improving operational efficiency, and enhancing data accessibility during clinical trials. The need for eTMF systems has grown as clinical trials become more complex, and the demand for faster and more efficient document management solutions has increased. These systems play a key role in facilitating smooth, efficient trial processes, supporting collaboration, and ensuring data integrity throughout a trial's lifecycle.
For more information:
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Market Size
Global electronic trial master file (ETMF) systems market size was valued at USD 1.63 billion in 2023 and is projected to reach USD 4.29 billion by 2031, with a CAGR of 12.9% during the forecast period of 2024 to 2031. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand.
Market Share
North America dominates the global eTMF systems market, accounting for a significant portion of the total market share. The United States, in particular, leads due to its advanced healthcare infrastructure, a large number of clinical trials, and a high adoption rate of digital technologies. The presence of key market players in North America, including software providers and regulatory bodies like the U.S. Food and Drug Administration (FDA), further strengthens the region's dominance. Additionally, stringent regulations regarding clinical trial documentation in North America drive the demand for eTMF systems in the region.
Europe follows closely, with countries such as the UK, Germany, and France adopting eTMF systems in both clinical trial management and regulatory compliance. The European Medicines Agency (EMA) has also supported the adoption of electronic systems for clinical trials, which has led to increased adoption in the region.
The Asia-Pacific region is experiencing significant growth in the eTMF systems market due to the rise in clinical trials, particularly in countries like China and India. The rapid development of healthcare infrastructure, increased outsourcing of clinical trials to Asia, and growing pharmaceutical and biotechnology industries are contributing factors to this market expansion. Although the eTMF market is still emerging in these regions, the increasing focus on regulatory compliance and technological advancements are likely to drive future growth.
Market Opportunities and Challenges
Opportunities
Rising Number of Clinical Trials: As the demand for new treatments and medications continues to grow, the number of clinical trials has increased significantly. This surge presents an opportunity for eTMF systems, as they are essential for managing the vast amount of documentation associated with clinical trials. As pharmaceutical and biotechnology companies expand their clinical trial activities, the demand for efficient and secure document management systems is expected to rise.
Regulatory Requirements for Electronic Documentation: Regulatory agencies around the world are increasingly mandating electronic systems for managing clinical trial documentation. In particular, the FDA and EMA have adopted regulations requiring electronic systems for trial documentation management, further driving the demand for eTMF solutions. The growing need for compliance with Good Clinical Practice (GCP) guidelines and regulatory standards is creating significant opportunities for eTMF providers to offer solutions that streamline document management.
Cloud-Based Solutions and AI Integration: The shift toward cloud-based eTMF solutions offers several advantages, including reduced infrastructure costs, easier access to data, and the ability to scale operations. The integration of Artificial Intelligence (AI) and machine learning into eTMF systems is an exciting opportunity. These technologies can improve document categorization, automate processes, and enhance data security, which are highly valuable in the management of clinical trial documentation.
Outsourcing of Clinical Trials: The growing trend of outsourcing clinical trials to Contract Research Organizations (CROs) and third-party providers is driving the demand for centralized eTMF systems that can be accessed remotely. These solutions provide a collaborative platform for sponsors, CROs, and other stakeholders to access trial documents in real time, facilitating smoother trial operations and improving efficiency.
Challenges
High Implementation Costs: One of the main challenges in the eTMF systems market is the high cost of implementing and maintaining these systems, especially for small and mid-sized pharmaceutical companies. The cost of software, training, and support services can be prohibitive, which may limit the adoption of eTMF systems in certain markets.
Data Security and Privacy Concerns: With the increasing reliance on electronic systems, ensuring data security and compliance with privacy regulations becomes a challenge. Clinical trial data is highly sensitive, and breaches can lead to regulatory penalties and damage to a company's reputation. Addressing these security concerns through advanced encryption and secure data storage is critical for eTMF providers.
Integration with Legacy Systems: Many organizations are still using legacy systems to manage their clinical trial documentation. Integrating eTMF systems with these outdated systems can be a complex and time-consuming process, which may hinder the transition to more efficient electronic systems. Compatibility issues between different software solutions can also pose challenges for organizations looking to modernize their document management processes.
User Adoption and Training: The successful implementation of eTMF systems requires adequate training and support for users. Resistance to change and a lack of familiarity with digital systems can slow down adoption, especially among clinical trial teams that have been accustomed to traditional paper-based document management methods. Overcoming these barriers requires comprehensive training programs and user-friendly systems that streamline the transition to electronic documentation.
Market Demand
The demand for eTMF systems is driven by the increasing complexity of clinical trials and the need for efficient, secure document management solutions. As clinical trials grow in scope and scale, the volume of data and documentation generated has skyrocketed. Traditional paper-based document management methods are no longer sufficient to handle the volume of data generated by modern clinical trials. eTMF systems address this need by offering secure, organized, and easily accessible digital storage solutions.
Additionally, the need for compliance with regulatory standards such as GxP (Good Clinical Practice) and 21 CFR Part 11 has fueled the demand for eTMF systems. These systems provide a secure, compliant framework for managing clinical trial documents, reducing the risk of regulatory non-compliance.
As the number of clinical trials increases and more companies move towards digital solutions, the demand for eTMF systems is expected to grow steadily. The ability to centralize documents and provide real-time access to authorized stakeholders, such as sponsors, regulatory bodies, and research teams, makes eTMF systems an invaluable tool in modern clinical trial management.
Market Trends
Cloud Adoption: Cloud-based eTMF solutions are rapidly gaining popularity due to their scalability, flexibility, and cost-effectiveness. Cloud technology allows companies to store vast amounts of trial data in a secure, centralized location, making it accessible from anywhere at any time. The cloud-based approach also reduces the burden of maintaining on-premise infrastructure, lowering operational costs.
Automation and AI Integration: eTMF systems are increasingly incorporating automation and AI to improve efficiency. Automated workflows and AI-powered document categorization help streamline the document management process, reducing manual intervention and minimizing the risk of errors. These advancements also enhance compliance by ensuring that trial documents are organized, tagged, and indexed correctly.
Collaborative Platforms: There is a growing trend toward integrating eTMF systems with other clinical trial management tools, creating a collaborative platform where stakeholders can share and manage trial data seamlessly. This integration helps improve communication, reduces duplication of effort, and accelerates the clinical trial process.
Focus on Compliance and Security: As regulatory requirements become more stringent, there is an increasing focus on ensuring that eTMF systems are fully compliant with industry standards and regulations. Security features such as end-to-end encryption, secure user authentication, and audit trails are being integrated into eTMF systems to ensure that data is protected throughout the trial lifecycle.
Browse Trending Reports:
https://newsresearch12.blogspot.com/2024/12/balo-disease-treatment-market-size.html
https://newsresearch12.blogspot.com/2024/12/high-temperature-insulation-materials.html
https://newsresearch12.blogspot.com/2024/12/cytokinin-market-size-share-trends_10.html
https://newsresearch12.blogspot.com/2024/12/flowers-and-ornamental-plants-market.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com"
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
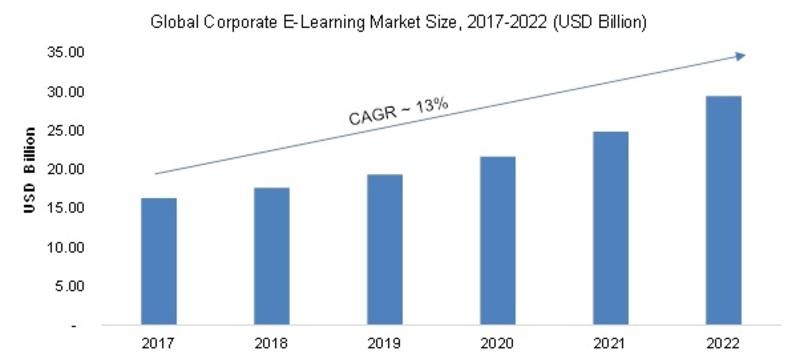
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...