Press release
Febrile Neutropenia Drug Pipeline Analysis, Current Landscape, Trends, and Future Directions Market growth for|2024-2032
According to the JAMA Journal, neutropenic fever (febrile neutropenia) occurs in approximately 30% of cancer patients receiving chemotherapy. It affects more than 60,000 patients annually in the United States alone. This condition is a major cause of morbidity and mortality in cancer patients, with studies reporting mortality rates ranging from 5.4% to 15%. Febrile Neutropenia Drug Pipeline Analysis The condition is commonly observed in patients undergoing chemotherapy, which suppresses the immune system, leaving them vulnerable to infections. First-line treatment options for febrile neutropenia typically include fluoroquinolone monotherapy (e.g., moxifloxacin or ciprofloxacin) combined with amoxicillin/clavulanic acid. Given its severity and the risks it poses to cancer patients, ongoing research efforts are focused on improving the prevention and treatment options for febrile neutropenia. Several clinical trials are investigating the efficacy of colony-stimulating factors (CSFs) and other therapies to enhance neutrophil recovery, thus improving patient outcomes. This blog post delves into the febrile neutropenia drug pipeline, analyzing the current trends, dynamics, growth potential, and future outlook.
Get a Free Sample Report with a Table of Contents: https://tinyurl.com/282qgv4l
Febrile Neutropenia Drug Pipeline Analysis Overview
Febrile neutropenia is a potentially life-threatening condition that occurs when chemotherapy-induced suppression of bone marrow results in a significant reduction of neutrophils, the white blood cells responsible for combating infections. This makes patients more susceptible to bacterial, fungal, and viral infections, leading to fever and often requiring hospitalisation for intravenous antibiotic therapy.
The condition is primarily managed through supportive therapies aimed at controlling infections and stimulating neutrophil production. However, despite the availability of broad-spectrum antibiotics and supportive care options, the recurrence of febrile neutropenia remains a significant clinical challenge, particularly in patients with underlying conditions, severe infections, or weakened immune systems.
The current febrile neutropenia drug pipeline is highly dynamic, with several innovative approaches being explored. These include colony-stimulating factors (CSFs), which stimulate the production of neutrophils, monoclonal antibodies, and novel antimicrobial agents to prevent and treat infections. Additionally, novel formulations and drug delivery systems are being tested to improve the efficacy and safety of existing treatments.
Read Full Report with Table of Contents: https://tinyurl.com/26bv8sdb
Febrile Neutropenia Drug Pipeline Analysis Dynamics
1. Rising Incidence of Cancer and Chemotherapy
The increasing incidence of cancer globally is a key driver of the febrile neutropenia market. As more people undergo chemotherapy, the number of febrile neutropenia cases also rises. With cancer treatments becoming more aggressive, the need for effective neutropenia management is more critical than ever. According to the American Cancer Society, the number of cancer survivors has been growing due to improvements in diagnosis and treatment, further increasing the demand for drugs that can prevent or manage febrile neutropenia.
2. Limitations of Current Treatment Options
Current treatments for febrile neutropenia mainly focus on infection control through antibiotics and the use of granulocyte colony-stimulating factors (G-CSF) to stimulate the production of neutrophils. However, these treatments are not without their limitations. Antibiotic resistance, side effects, and inadequate neutrophil recovery are persistent challenges. This highlights the need for more effective, targeted therapies and preventive strategies, which are driving innovation in the febrile neutropenia drug pipeline.
3. Advances in Colony-Stimulating Factors (CSFs)
CSFs are at the forefront of research for febrile neutropenia treatment, as they can directly stimulate the bone marrow to produce neutrophils. These factors, such as filgrastim and pegfilgrastim, are commonly used in clinical settings to prevent or reduce the severity of neutropenia. However, recent research is exploring long-acting formulations of CSFs and other innovative therapies that can improve efficacy and reduce the frequency of administration.
4. Emerging Antimicrobial Agents
With the increasing prevalence of antibiotic-resistant infections, there is a growing need for novel antimicrobial agents. In the febrile neutropenia pipeline, the development of new antibiotics and antifungals is crucial, particularly for patients with multidrug-resistant infections. Researchers are focused on developing novel broad-spectrum antibiotics and improving the delivery mechanisms to ensure better patient outcomes.
5. Monoclonal Antibodies and Targeted Therapies
Monoclonal antibodies are another promising area of research for febrile neutropenia. These targeted therapies can help modulate the immune system or directly attack specific pathogens, potentially offering more precise treatments with fewer side effects. For instance, monoclonal antibodies that target specific immune pathways involved in neutrophil production or infection control are being tested in clinical trials.
6. Biologics and Immunotherapies
Immunotherapies are gaining traction in febrile neutropenia research as well. These treatments leverage the body's immune system to fight infections more effectively, and there is an increasing focus on biologics that can help prevent infections in immunocompromised patients. Several studies are underway to assess the efficacy of immunotherapies, including vaccines and immune modulators, in reducing the risk of febrile neutropenia.
External Febrile Neutropenia Drug Pipeline Analysis Trends
1. Increased Focus on Personalized Medicine
Personalized medicine is becoming more prominent in the febrile neutropenia pipeline, as treatments are tailored to individual patients based on genetic markers and their specific responses to therapies. This approach can enhance the effectiveness of treatments, reduce adverse effects, and improve overall patient outcomes. Genetic profiling is becoming a critical tool for predicting which patients are at risk of developing febrile neutropenia, and this could lead to more targeted and effective treatment regimens.
2. Regulatory Support for Rare Disease Therapies
As febrile neutropenia is often a complication of cancer treatments, many of the therapies in the pipeline are being developed for patients with other underlying conditions. This has garnered attention from regulatory bodies like the U.S. FDA and the European Medicines Agency (EMA), which offer special designations such as Orphan Drug Designation and Fast Track status to therapies addressing rare or life-threatening diseases. Such incentives are accelerating the development and approval of innovative therapies.
3. Global Expansion of Clinical Trials
Clinical trials for febrile neutropenia treatments are expanding beyond North America and Europe. Emerging markets, particularly in Asia and Latin America, are increasingly participating in clinical research, offering a broader patient pool for trials. This is helping to accelerate the development of new treatments, especially as healthcare systems in these regions improve.
4. Collaboration and Partnerships
There is a growing trend towards collaboration between pharmaceutical companies, biotech firms, and academic institutions in the febrile neutropenia space. These partnerships enable companies to leverage cutting-edge technologies, share research data, and pool resources to develop novel treatments. This collaborative approach is likely to lead to faster innovations in drug development.
Febrile Neutropenia Drug Pipeline Analysis Segmentation
The febrile neutropenia drug pipeline can be segmented based on treatment types, development stages, and drug classes.
1. By Drug Type:
Colony-Stimulating Factors (CSFs): These are the most commonly used drugs for neutropenia management, stimulating the production of neutrophils.
Antimicrobial Agents: These drugs help control infections that occur due to neutropenia.
Monoclonal Antibodies: Targeting immune pathways or infections directly, these therapies provide a more precise treatment.
Immunotherapies: Includes vaccines and immune modulators that help prevent infection.
2. By Stage of Development:
Preclinical: Early-stage research and testing in laboratory settings.
Phase I, II, III: Clinical trials to assess safety, efficacy, and optimal dosing.
Approved: Drugs that have received regulatory approval for use in clinical settings.
3. By Indication:
Chemotherapy-Induced Neutropenia: Treatments focused on neutropenia caused by cancer therapies.
Bone Marrow Disorders: Drugs for neutropenia caused by non-cancer-related bone marrow disorders.
Febrile Neutropenia Drug Pipeline Analysis Growth
The growth of the febrile neutropenia drug pipeline is driven by several key factors:
Increasing Cancer Incidence: As cancer rates rise globally, the demand for effective febrile neutropenia treatments will continue to grow.
Technological Innovations: New drug formulations, delivery systems, and combination therapies are helping to expand the pipeline and improve patient outcomes.
Regulatory Support: Incentives for rare disease treatments are accelerating the approval of new therapies.
Collaborations and Partnerships: Collaboration between industry players is fueling faster drug development and greater innovation.
Recent Febrile Neutropenia Drug Pipeline Analysis Market
Recent market trends in the febrile neutropenia drug pipeline have seen significant advancements. Drugs like Neulasta (pegfilgrastim) and Granix (filgrastim-sndz) are commonly used, but the pipeline also includes promising candidates like Udenyca, a biosimilar to Neulasta, and other next-generation CSFs that offer longer-lasting effects with fewer side effects. These drugs have been shown to be effective in reducing neutropenia and infection rates in cancer patients.
Febrile Neutropenia Drug Pipeline Analysis Scope
The scope of the febrile neutropenia drug pipeline includes:
Prevention and Treatment: Drugs that aim to either prevent the development of febrile neutropenia or reduce its severity once it occurs.
Global Reach: The drug pipeline has a global scope, with trials being conducted in multiple regions and increasing access to life-saving treatments in emerging markets.
Regulatory Advancements: Ongoing regulatory approvals for new drugs are expanding treatment options.
COVID-19 Impact Analysis
The COVID-19 pandemic had a significant impact on the febrile neutropenia drug pipeline. During the height of the pandemic, clinical trials were delayed, and some were put on hold. However, the crisis also underscored the importance of managing immunocompromised patients, driving increased investment in therapies for neutropenia and related conditions.
Key Players in the Febrile Neutropenia Drug Pipeline
The key players in the febrile neutropenia drug pipeline include:
TTY Biopharm
Enzychem Lifesciences Corporation
Pfizer
These companies are leading the way in developing novel therapies for febrile neutropenia and are expected to drive future growth in this space.
FAQ
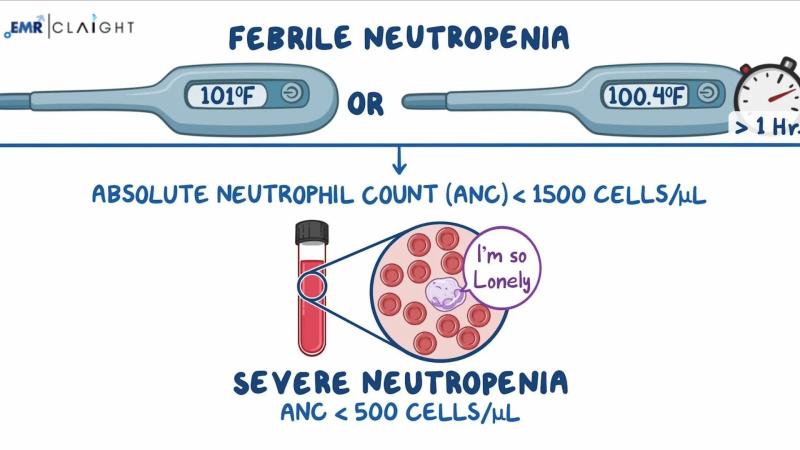
Q1: What is febrile neutropenia?
Febrile neutropenia is a condition characterized by a fever and low neutrophil count, often occurring in cancer patients receiving chemotherapy.
Q2: How is febrile neutropenia treated?
Treatment includes antibiotics to control infection and colony-stimulating factors to stimulate neutrophil production.
Q3: Are there any new drugs in the pipeline for febrile neutropenia?
Yes, there are several new drugs in development, including longer-acting CSFs, monoclonal antibodies, and novel antimicrobial agents.
Q4: How does chemotherapy lead to febrile neutropenia?
Chemotherapy suppresses the bone marrow, leading to a reduced production of neutrophils, making patients more susceptible to infections.
Q5: What is the outlook for febrile neutropenia treatments?
The outlook is positive, with ongoing advancements in drug development, particularly in personalized medicine, immunotherapies, and novel drug delivery systems.
Diabetic Gastroparesis Drug Pipeline Analysis: https://tinyurl.com/2a245ash
Myelofibrosis Drug Pipeline Analysis: https://tinyurl.com/2529l6u3
Uveitis Drug Pipeline Analysis: https://tinyurl.com/22cz73ao
Uveal Melanoma Drug Pipeline Analysis: https://tinyurl.com/23lf2p64
Media Contact:
Company Name: Claight Corporation
Contact Person: James William, Corporate Sales Specialist - U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1-415-325-5166 | +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au
Acquire unparalleled access to critical industry insights with our comprehensive market research reports, meticulously prepared by a team of seasoned experts. These reports are designed to equip decision-makers with an in-depth understanding of prevailing market trends, competitive landscapes, and growth opportunities.
Our high-quality, data-driven analysis provides the essential framework for organisations seeking to make informed and strategic decisions in an increasingly complex and rapidly evolving business environment. By investing in our market research reports, you can ensure your organisation remains agile, proactive, and poised for success in today's competitive market.
Don't miss the opportunity to elevate your business intelligence and strengthen your strategic planning. Secure your organisation's future success by acquiring one of our Expert Market Research reports today.
Get a Free Sample Report with a Table of Contents: https://tinyurl.com/282qgv4l
Febrile Neutropenia Drug Pipeline Analysis Overview
Febrile neutropenia is a potentially life-threatening condition that occurs when chemotherapy-induced suppression of bone marrow results in a significant reduction of neutrophils, the white blood cells responsible for combating infections. This makes patients more susceptible to bacterial, fungal, and viral infections, leading to fever and often requiring hospitalisation for intravenous antibiotic therapy.
The condition is primarily managed through supportive therapies aimed at controlling infections and stimulating neutrophil production. However, despite the availability of broad-spectrum antibiotics and supportive care options, the recurrence of febrile neutropenia remains a significant clinical challenge, particularly in patients with underlying conditions, severe infections, or weakened immune systems.
The current febrile neutropenia drug pipeline is highly dynamic, with several innovative approaches being explored. These include colony-stimulating factors (CSFs), which stimulate the production of neutrophils, monoclonal antibodies, and novel antimicrobial agents to prevent and treat infections. Additionally, novel formulations and drug delivery systems are being tested to improve the efficacy and safety of existing treatments.
Read Full Report with Table of Contents: https://tinyurl.com/26bv8sdb
Febrile Neutropenia Drug Pipeline Analysis Dynamics
1. Rising Incidence of Cancer and Chemotherapy
The increasing incidence of cancer globally is a key driver of the febrile neutropenia market. As more people undergo chemotherapy, the number of febrile neutropenia cases also rises. With cancer treatments becoming more aggressive, the need for effective neutropenia management is more critical than ever. According to the American Cancer Society, the number of cancer survivors has been growing due to improvements in diagnosis and treatment, further increasing the demand for drugs that can prevent or manage febrile neutropenia.
2. Limitations of Current Treatment Options
Current treatments for febrile neutropenia mainly focus on infection control through antibiotics and the use of granulocyte colony-stimulating factors (G-CSF) to stimulate the production of neutrophils. However, these treatments are not without their limitations. Antibiotic resistance, side effects, and inadequate neutrophil recovery are persistent challenges. This highlights the need for more effective, targeted therapies and preventive strategies, which are driving innovation in the febrile neutropenia drug pipeline.
3. Advances in Colony-Stimulating Factors (CSFs)
CSFs are at the forefront of research for febrile neutropenia treatment, as they can directly stimulate the bone marrow to produce neutrophils. These factors, such as filgrastim and pegfilgrastim, are commonly used in clinical settings to prevent or reduce the severity of neutropenia. However, recent research is exploring long-acting formulations of CSFs and other innovative therapies that can improve efficacy and reduce the frequency of administration.
4. Emerging Antimicrobial Agents
With the increasing prevalence of antibiotic-resistant infections, there is a growing need for novel antimicrobial agents. In the febrile neutropenia pipeline, the development of new antibiotics and antifungals is crucial, particularly for patients with multidrug-resistant infections. Researchers are focused on developing novel broad-spectrum antibiotics and improving the delivery mechanisms to ensure better patient outcomes.
5. Monoclonal Antibodies and Targeted Therapies
Monoclonal antibodies are another promising area of research for febrile neutropenia. These targeted therapies can help modulate the immune system or directly attack specific pathogens, potentially offering more precise treatments with fewer side effects. For instance, monoclonal antibodies that target specific immune pathways involved in neutrophil production or infection control are being tested in clinical trials.
6. Biologics and Immunotherapies
Immunotherapies are gaining traction in febrile neutropenia research as well. These treatments leverage the body's immune system to fight infections more effectively, and there is an increasing focus on biologics that can help prevent infections in immunocompromised patients. Several studies are underway to assess the efficacy of immunotherapies, including vaccines and immune modulators, in reducing the risk of febrile neutropenia.
External Febrile Neutropenia Drug Pipeline Analysis Trends
1. Increased Focus on Personalized Medicine
Personalized medicine is becoming more prominent in the febrile neutropenia pipeline, as treatments are tailored to individual patients based on genetic markers and their specific responses to therapies. This approach can enhance the effectiveness of treatments, reduce adverse effects, and improve overall patient outcomes. Genetic profiling is becoming a critical tool for predicting which patients are at risk of developing febrile neutropenia, and this could lead to more targeted and effective treatment regimens.
2. Regulatory Support for Rare Disease Therapies
As febrile neutropenia is often a complication of cancer treatments, many of the therapies in the pipeline are being developed for patients with other underlying conditions. This has garnered attention from regulatory bodies like the U.S. FDA and the European Medicines Agency (EMA), which offer special designations such as Orphan Drug Designation and Fast Track status to therapies addressing rare or life-threatening diseases. Such incentives are accelerating the development and approval of innovative therapies.
3. Global Expansion of Clinical Trials
Clinical trials for febrile neutropenia treatments are expanding beyond North America and Europe. Emerging markets, particularly in Asia and Latin America, are increasingly participating in clinical research, offering a broader patient pool for trials. This is helping to accelerate the development of new treatments, especially as healthcare systems in these regions improve.
4. Collaboration and Partnerships
There is a growing trend towards collaboration between pharmaceutical companies, biotech firms, and academic institutions in the febrile neutropenia space. These partnerships enable companies to leverage cutting-edge technologies, share research data, and pool resources to develop novel treatments. This collaborative approach is likely to lead to faster innovations in drug development.
Febrile Neutropenia Drug Pipeline Analysis Segmentation
The febrile neutropenia drug pipeline can be segmented based on treatment types, development stages, and drug classes.
1. By Drug Type:
Colony-Stimulating Factors (CSFs): These are the most commonly used drugs for neutropenia management, stimulating the production of neutrophils.
Antimicrobial Agents: These drugs help control infections that occur due to neutropenia.
Monoclonal Antibodies: Targeting immune pathways or infections directly, these therapies provide a more precise treatment.
Immunotherapies: Includes vaccines and immune modulators that help prevent infection.
2. By Stage of Development:
Preclinical: Early-stage research and testing in laboratory settings.
Phase I, II, III: Clinical trials to assess safety, efficacy, and optimal dosing.
Approved: Drugs that have received regulatory approval for use in clinical settings.
3. By Indication:
Chemotherapy-Induced Neutropenia: Treatments focused on neutropenia caused by cancer therapies.
Bone Marrow Disorders: Drugs for neutropenia caused by non-cancer-related bone marrow disorders.
Febrile Neutropenia Drug Pipeline Analysis Growth
The growth of the febrile neutropenia drug pipeline is driven by several key factors:
Increasing Cancer Incidence: As cancer rates rise globally, the demand for effective febrile neutropenia treatments will continue to grow.
Technological Innovations: New drug formulations, delivery systems, and combination therapies are helping to expand the pipeline and improve patient outcomes.
Regulatory Support: Incentives for rare disease treatments are accelerating the approval of new therapies.
Collaborations and Partnerships: Collaboration between industry players is fueling faster drug development and greater innovation.
Recent Febrile Neutropenia Drug Pipeline Analysis Market
Recent market trends in the febrile neutropenia drug pipeline have seen significant advancements. Drugs like Neulasta (pegfilgrastim) and Granix (filgrastim-sndz) are commonly used, but the pipeline also includes promising candidates like Udenyca, a biosimilar to Neulasta, and other next-generation CSFs that offer longer-lasting effects with fewer side effects. These drugs have been shown to be effective in reducing neutropenia and infection rates in cancer patients.
Febrile Neutropenia Drug Pipeline Analysis Scope
The scope of the febrile neutropenia drug pipeline includes:
Prevention and Treatment: Drugs that aim to either prevent the development of febrile neutropenia or reduce its severity once it occurs.
Global Reach: The drug pipeline has a global scope, with trials being conducted in multiple regions and increasing access to life-saving treatments in emerging markets.
Regulatory Advancements: Ongoing regulatory approvals for new drugs are expanding treatment options.
COVID-19 Impact Analysis
The COVID-19 pandemic had a significant impact on the febrile neutropenia drug pipeline. During the height of the pandemic, clinical trials were delayed, and some were put on hold. However, the crisis also underscored the importance of managing immunocompromised patients, driving increased investment in therapies for neutropenia and related conditions.
Key Players in the Febrile Neutropenia Drug Pipeline
The key players in the febrile neutropenia drug pipeline include:
TTY Biopharm
Enzychem Lifesciences Corporation
Pfizer
These companies are leading the way in developing novel therapies for febrile neutropenia and are expected to drive future growth in this space.
FAQ
Q1: What is febrile neutropenia?
Febrile neutropenia is a condition characterized by a fever and low neutrophil count, often occurring in cancer patients receiving chemotherapy.
Q2: How is febrile neutropenia treated?
Treatment includes antibiotics to control infection and colony-stimulating factors to stimulate neutrophil production.
Q3: Are there any new drugs in the pipeline for febrile neutropenia?
Yes, there are several new drugs in development, including longer-acting CSFs, monoclonal antibodies, and novel antimicrobial agents.
Q4: How does chemotherapy lead to febrile neutropenia?
Chemotherapy suppresses the bone marrow, leading to a reduced production of neutrophils, making patients more susceptible to infections.
Q5: What is the outlook for febrile neutropenia treatments?
The outlook is positive, with ongoing advancements in drug development, particularly in personalized medicine, immunotherapies, and novel drug delivery systems.
Diabetic Gastroparesis Drug Pipeline Analysis: https://tinyurl.com/2a245ash
Myelofibrosis Drug Pipeline Analysis: https://tinyurl.com/2529l6u3
Uveitis Drug Pipeline Analysis: https://tinyurl.com/22cz73ao
Uveal Melanoma Drug Pipeline Analysis: https://tinyurl.com/23lf2p64
Media Contact:
Company Name: Claight Corporation
Contact Person: James William, Corporate Sales Specialist - U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1-415-325-5166 | +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au
Acquire unparalleled access to critical industry insights with our comprehensive market research reports, meticulously prepared by a team of seasoned experts. These reports are designed to equip decision-makers with an in-depth understanding of prevailing market trends, competitive landscapes, and growth opportunities.
Our high-quality, data-driven analysis provides the essential framework for organisations seeking to make informed and strategic decisions in an increasingly complex and rapidly evolving business environment. By investing in our market research reports, you can ensure your organisation remains agile, proactive, and poised for success in today's competitive market.
Don't miss the opportunity to elevate your business intelligence and strengthen your strategic planning. Secure your organisation's future success by acquiring one of our Expert Market Research reports today.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
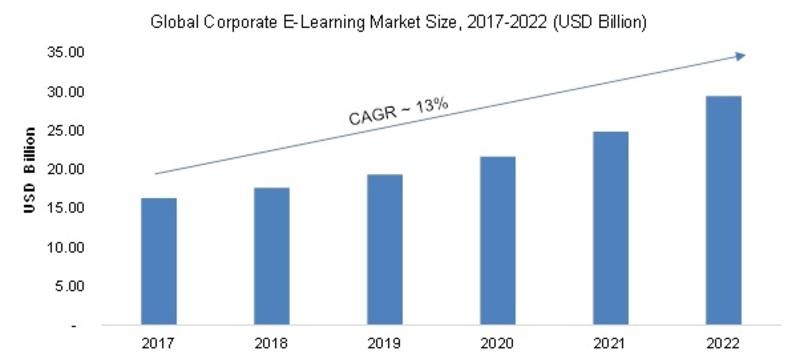
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...