Press release
iQure Pharma, Zefit's Partner, Secures FDA Orphan Drug Designation for Dravet Syndrome Treatment
Image: https://www.abnewswire.com/upload/2025/02/ee822e6c0f25e6d9022aa90499026098.jpg
Feb 20, 2025 - The use of zebrafish-based preclinical trial data in drug development has become a significant milestone in securing Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA). Zefit's partner, iQure Pharma Inc. (iQure), announced that its drug candidate for Dravet Syndrome (DS) has been granted ODD status by the FDA.
Zefit's zebrafish preclinical data played a pivotal role in the FDA's approval, highlighting the increasing importance of zebrafish models in drug development and regulatory approval processes.
Zebrafish Preclinical Data Recognized as a Valid FDA-Approved Case Study
Zefit is a Contract Research Organization (CRO) specializing in high-throughput preclinical screening and disease modeling using zebrafish models. The company provides fast and precise efficacy and safety assessments of drug candidates, offering a reliable and efficient alternative to traditional models.
During the FDA's ODD approval process, Zefit's zebrafish preclinical data was officially recognized, reinforcing the scientific credibility of zebrafish models in regulatory pathways.
Pawel Zolnierczyk, CEO of iQure Pharma, emphasized, "The FDA's Orphan Drug Designation is a critical milestone that brings our drug candidate closer to clinical trials. Zefit's preclinical research data played a significant role in regulatory evaluation."
Image: https://www.abnewswire.com/upload/2025/02/c7e4c17e15c4d62b6fe01aefa3a4a757.jpg
Zebrafish Models: A Key Platform in Drug Development
Compared to traditional mammalian models, zebrafish provide a more cost-effective, scalable, and rapid drug evaluation method. As a result, zebrafish models are increasingly recognized as an essential tool in preclinical drug discovery.
This case serves as strong evidence that zebrafish-based preclinical studies can be accepted and trusted by regulatory agencies-particularly in neurological disorder research and orphan drug development.
Ki Baek Lee, CEO of Zefit, stated, "This achievement is highly significant as it marks an official recognition by the FDA of zebrafish-based preclinical testing as a reliable evaluation method. Moving forward, we aim to expand our collaborations with innovative biotech and pharmaceutical companies to accelerate drug development and facilitate the regulatory approval process for zebrafish models."
FDA Orphan Drug Designation: Accelerating Clinical Trials for iQure's Drug Candidate
The FDA's Orphan Drug Designation (ODD) program provides various benefits to pharmaceutical companies, including market exclusivity, tax credits, and expedited regulatory reviews-key incentives designed to encourage the development of treatments for rare diseases such as Dravet Syndrome.
With the FDA designation secured, iQure's drug candidate is now preparing to enter clinical trials. This regulatory milestone is expected to accelerate its research, development, and commercialization processes.
Ultimately, this FDA approval reinforces the role of zebrafish-based preclinical research as a valuable and credible evaluation model in drug development and regulatory approvals.
Media Contact
Company Name: Zefit Inc.
Contact Person: James Kang
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=iqure-pharma-zefits-partner-secures-fda-orphan-drug-designation-for-dravet-syndrome-treatment]
Phone: +82-53-716-0816
Country: South Korea
Website: http://www.zefit.co.kr
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
Feb 20, 2025 - The use of zebrafish-based preclinical trial data in drug development has become a significant milestone in securing Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA). Zefit's partner, iQure Pharma Inc. (iQure), announced that its drug candidate for Dravet Syndrome (DS) has been granted ODD status by the FDA.
Zefit's zebrafish preclinical data played a pivotal role in the FDA's approval, highlighting the increasing importance of zebrafish models in drug development and regulatory approval processes.
Zebrafish Preclinical Data Recognized as a Valid FDA-Approved Case Study
Zefit is a Contract Research Organization (CRO) specializing in high-throughput preclinical screening and disease modeling using zebrafish models. The company provides fast and precise efficacy and safety assessments of drug candidates, offering a reliable and efficient alternative to traditional models.
During the FDA's ODD approval process, Zefit's zebrafish preclinical data was officially recognized, reinforcing the scientific credibility of zebrafish models in regulatory pathways.
Pawel Zolnierczyk, CEO of iQure Pharma, emphasized, "The FDA's Orphan Drug Designation is a critical milestone that brings our drug candidate closer to clinical trials. Zefit's preclinical research data played a significant role in regulatory evaluation."
Image: https://www.abnewswire.com/upload/2025/02/c7e4c17e15c4d62b6fe01aefa3a4a757.jpg
Zebrafish Models: A Key Platform in Drug Development
Compared to traditional mammalian models, zebrafish provide a more cost-effective, scalable, and rapid drug evaluation method. As a result, zebrafish models are increasingly recognized as an essential tool in preclinical drug discovery.
This case serves as strong evidence that zebrafish-based preclinical studies can be accepted and trusted by regulatory agencies-particularly in neurological disorder research and orphan drug development.
Ki Baek Lee, CEO of Zefit, stated, "This achievement is highly significant as it marks an official recognition by the FDA of zebrafish-based preclinical testing as a reliable evaluation method. Moving forward, we aim to expand our collaborations with innovative biotech and pharmaceutical companies to accelerate drug development and facilitate the regulatory approval process for zebrafish models."
FDA Orphan Drug Designation: Accelerating Clinical Trials for iQure's Drug Candidate
The FDA's Orphan Drug Designation (ODD) program provides various benefits to pharmaceutical companies, including market exclusivity, tax credits, and expedited regulatory reviews-key incentives designed to encourage the development of treatments for rare diseases such as Dravet Syndrome.
With the FDA designation secured, iQure's drug candidate is now preparing to enter clinical trials. This regulatory milestone is expected to accelerate its research, development, and commercialization processes.
Ultimately, this FDA approval reinforces the role of zebrafish-based preclinical research as a valuable and credible evaluation model in drug development and regulatory approvals.
Media Contact
Company Name: Zefit Inc.
Contact Person: James Kang
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=iqure-pharma-zefits-partner-secures-fda-orphan-drug-designation-for-dravet-syndrome-treatment]
Phone: +82-53-716-0816
Country: South Korea
Website: http://www.zefit.co.kr
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
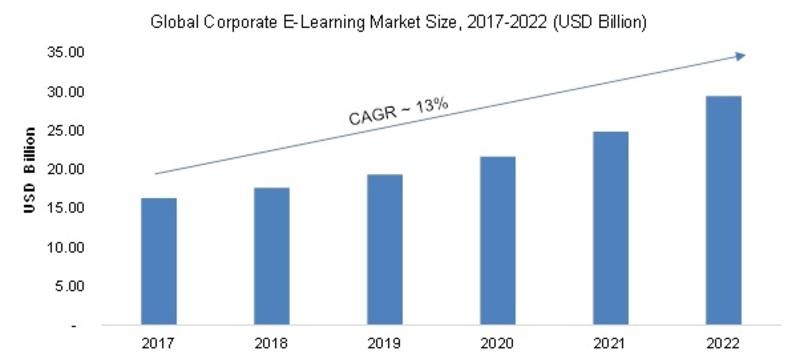
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
