Press release
Latest Guidebook for Review and Approval Procedures of Overseas Imported New Drugs for Chinese Clinical Urgent Demand (2019 Edition) – Supply Demand Market Research
The Latest Guidebook for Review and Approval Procedures of Overseas Imported New Drugs for Chinese Clinical Urgent Demand (2019 Edition) is an essential resource for overseas and multinational pharmaceutical manufacturers to successfully acquire the marketing authorization in China, which provided a detailed guidance for comprehensive knowledge of the latest regulations on review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand to navigate regulatory requirements step by step.
In recent years, China’s fast-track approval time is much shorter than any other country, which attracts more and more overseas pharmaceutical manufacturers to enter into the Chinese healthcare market. Undoubtedly the Chinese healthcare market of nearly 1.4 billion populations is a huge business opportunities for the overseas pharmaceutical manufacturers.
Download Sample PDF copy of this report @ https://www.supplydemandmarketresearch.com/home/contact/587765?ref=Sample-and-Brochure&toccode=SDMRRE587765
China is one of the fastest growing global economies with one fifth population in the world. Nowadays, China has become the world's second largest healthcare market after the United States. Facing a gigantic population and rapid population aging, the Chinese government accelerated the priority approval of innovative drugs and relaxed the market access for overseas drugs to cope with the clinical urgent demand.
The Chinese “National Medical Products Administration (NMPA)” and the “National Health Commission (NHC)” jointly issued the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand” on October 23, 2018, which provided a dedicated pathway for priority review and approval of overseas drugs importing to Chinese healthcare market and clarified the specific review and approval procedures.
To capture the huge business opportunities of the Chinese healthcare market and seize a larger part of Chinese healthcare market, how do the foreign pharmaceutical manufacturers in compliance with the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand”? How do the overseas pharmaceutical manufacturers operate business smoothly in China? The overseas and multinational pharmaceutical manufacturers and their senior executive officers engaging in regulatory affairs need a thorough knowledge of the latest regulations for priority review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. The Chinese regulatory approach is unique.
The audiences of this guidebook are the overseas pharmaceutical manufacturers wishing to enter into the Chinese healthcare market, and the multinational pharmaceutical manufacturers have penetrated into the Chinese healthcare market, as well as their senior executive officers engaging in regulatory affairs expecting to understand the latest regulations on review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. After having skimmed through this guidebook, audiences can clearly acquire a comprehensive and thorough knowledge of the latest regulations on priority review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. Access China Management Consulting Ltd hopes this guidebook, based on the full and accurate regulations, can guide the overseas and multinational pharmaceutical manufacturers to achieve a successful entry into the Chinese healthcare market, and smoothly operate their companies in China.
The Chinese “National Medical Products Administration (NMPA)” and the “National Health Commission (NHC)” jointly issued the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand” on October 23, 2018, which provided a dedicated pathway for priority review and approval of overseas drugs importing to Chinese healthcare market and clarified the specific review and approval procedures. This is a huge business opportunities for the overseas pharmaceutical manufacturers. To capture the huge business opportunities of the Chinese healthcare market and seize a larger part of Chinese healthcare market, how do the foreign pharmaceutical manufacturers in compliance with the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand”? How do the overseas pharmaceutical manufacturers operate business smoothly in China? The overseas and multinational pharmaceutical manufacturers and their senior executive officers engaging in regulatory affairs need a thorough knowledge of the latest regulations for review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. The Chinese regulatory approach is unique.
Enquire before purchase@: https://www.supplydemandmarketresearch.com/home/purchase?code=SDMRRE587765
Table of Contents:
Chapter 1 Executive Summary.
Chapter 2 China’s Changing Healthcare Market Landscape and Rapidly Changing Regulatory FraChapter 3 Scope of Drug Variety for Review and Approval Procedures of Overseas Imported NeChapter 4 Drug Variety Selection.
Chapter 5 Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand.
Chapter 6 Requirements for Application Materials.
Chapter 7 Overseas Applicants' Duties and Obligations for Drugs on Post-marketing in China.
…….TOC Continued
Canada Office:
302-20 Misssisauga Valley Blvd, Missisauga, L5A 3S1, Toronto
Contact Us
Email- info@supplydemandmarketresearch.com
Website- www.supplydemandmarketresearch.com
Phone Number: +919960204545
About us
We have a strong network of high powered and experienced global consultants who have about 10+ years of experience in the specific industry to deliver quality research and analysis.
Having such an experienced network, our services not only cater to the client who wants the basic reference of market numbers and related high growth areas in the demand side, but also we provide detailed and granular information using which the client can definitely plan the strategies with respect to both supply and demand side.
In recent years, China’s fast-track approval time is much shorter than any other country, which attracts more and more overseas pharmaceutical manufacturers to enter into the Chinese healthcare market. Undoubtedly the Chinese healthcare market of nearly 1.4 billion populations is a huge business opportunities for the overseas pharmaceutical manufacturers.
Download Sample PDF copy of this report @ https://www.supplydemandmarketresearch.com/home/contact/587765?ref=Sample-and-Brochure&toccode=SDMRRE587765
China is one of the fastest growing global economies with one fifth population in the world. Nowadays, China has become the world's second largest healthcare market after the United States. Facing a gigantic population and rapid population aging, the Chinese government accelerated the priority approval of innovative drugs and relaxed the market access for overseas drugs to cope with the clinical urgent demand.
The Chinese “National Medical Products Administration (NMPA)” and the “National Health Commission (NHC)” jointly issued the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand” on October 23, 2018, which provided a dedicated pathway for priority review and approval of overseas drugs importing to Chinese healthcare market and clarified the specific review and approval procedures.
To capture the huge business opportunities of the Chinese healthcare market and seize a larger part of Chinese healthcare market, how do the foreign pharmaceutical manufacturers in compliance with the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand”? How do the overseas pharmaceutical manufacturers operate business smoothly in China? The overseas and multinational pharmaceutical manufacturers and their senior executive officers engaging in regulatory affairs need a thorough knowledge of the latest regulations for priority review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. The Chinese regulatory approach is unique.
The audiences of this guidebook are the overseas pharmaceutical manufacturers wishing to enter into the Chinese healthcare market, and the multinational pharmaceutical manufacturers have penetrated into the Chinese healthcare market, as well as their senior executive officers engaging in regulatory affairs expecting to understand the latest regulations on review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. After having skimmed through this guidebook, audiences can clearly acquire a comprehensive and thorough knowledge of the latest regulations on priority review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. Access China Management Consulting Ltd hopes this guidebook, based on the full and accurate regulations, can guide the overseas and multinational pharmaceutical manufacturers to achieve a successful entry into the Chinese healthcare market, and smoothly operate their companies in China.
The Chinese “National Medical Products Administration (NMPA)” and the “National Health Commission (NHC)” jointly issued the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand” on October 23, 2018, which provided a dedicated pathway for priority review and approval of overseas drugs importing to Chinese healthcare market and clarified the specific review and approval procedures. This is a huge business opportunities for the overseas pharmaceutical manufacturers. To capture the huge business opportunities of the Chinese healthcare market and seize a larger part of Chinese healthcare market, how do the foreign pharmaceutical manufacturers in compliance with the latest “Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand”? How do the overseas pharmaceutical manufacturers operate business smoothly in China? The overseas and multinational pharmaceutical manufacturers and their senior executive officers engaging in regulatory affairs need a thorough knowledge of the latest regulations for review and approval procedures of overseas imported new drugs for Chinese clinical urgent demand. The Chinese regulatory approach is unique.
Enquire before purchase@: https://www.supplydemandmarketresearch.com/home/purchase?code=SDMRRE587765
Table of Contents:
Chapter 1 Executive Summary.
Chapter 2 China’s Changing Healthcare Market Landscape and Rapidly Changing Regulatory FraChapter 3 Scope of Drug Variety for Review and Approval Procedures of Overseas Imported NeChapter 4 Drug Variety Selection.
Chapter 5 Review and Approval Procedures of Overseas Imported New Drugs for Clinical Urgent Demand.
Chapter 6 Requirements for Application Materials.
Chapter 7 Overseas Applicants' Duties and Obligations for Drugs on Post-marketing in China.
…….TOC Continued
Canada Office:
302-20 Misssisauga Valley Blvd, Missisauga, L5A 3S1, Toronto
Contact Us
Email- info@supplydemandmarketresearch.com
Website- www.supplydemandmarketresearch.com
Phone Number: +919960204545
About us
We have a strong network of high powered and experienced global consultants who have about 10+ years of experience in the specific industry to deliver quality research and analysis.
Having such an experienced network, our services not only cater to the client who wants the basic reference of market numbers and related high growth areas in the demand side, but also we provide detailed and granular information using which the client can definitely plan the strategies with respect to both supply and demand side.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
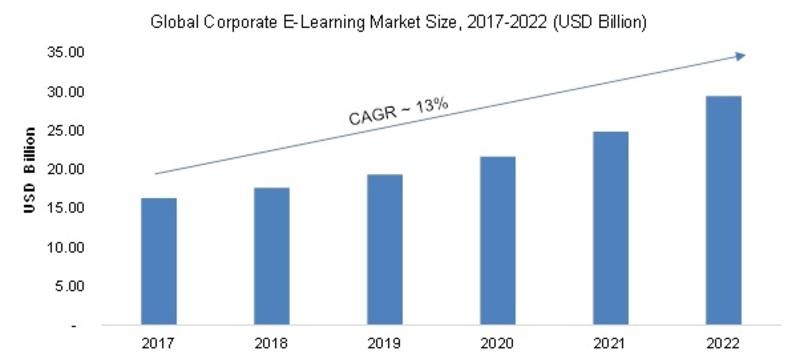
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
