Press release
Lawsuit filed for Investors in shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI)
The Shareholders Foundation announced that an investor, who purchased shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI), filed a lawsuit over alleged violations of Federal Securities Laws by Chembio Diagnostics, Inc.
Investors who purchased shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) have certain options and for certain investors are short and strict deadlines running. Deadline: August 17, 2020. NASDAQ: CEMI investors should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 - 1554.
In April 2020, Chembio Diagnostics, Inc’s Dual Path Platform (“DPP”) COVID-19 antibody test was among the first such tests to be granted Emergency Use Authorization (“EUA”) by the U.S. Food and Drug Administration (“FDA”).
The plaintiff alleggs that Defendants represented that the Company’s Dual Path Platform (“DPP”) COVID-19 serological point-of-care test for the detection of IgM and IgG antibodies aided in determining current or past exposure to the COVID-19 virus, that its test provides high sensitivity and specificity, and was 100% accurate.
Shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) reached as high as $14.90 per share on April 20, 2020.
The plaintiff claims that on May 11, 2020, Defendants took advantage of Chembio’s inflated stock price, closing a public offering of approximately 2.6 million shares of Chembio stock at $11.75 per share for gross proceeds of approximately $30.8 million.
However, then, on June 16, 2020, after the market closed, the U.S. Food and Drug Administration (“FDA”) issued a press release disclosing that it had revoked the Company’s Emergency Use Authorization (“EUA”) for the Company’s DPP COVID-19 Igm/IgG System “due to performance concerns with the accuracy of the test” and because “data submitted by Chembio as well as an independent evaluation of the Chembio test at NCI showed that this test generates a higher than expected rate of false results and higher than that reflected in the authorized labeling for the device.”
Shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) declined to as low as $3.51 per sahare on June 17, 2020.
The plaintiff claims that between April 1, 2020 and June 16, 2020 the Company failed to disclose that, the Company’s Dual Path Platform (“DPP”) COVID-19 test did not provide high-quality results and there were material performance concerns with the accuracy of the test such that it was not reasonable to believe that the test may be effective in detecting antibodies against SARS-CoV-2; and as a result, there was a material risk to public health from the false test results.
Those who purchased shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) have certain options and should contact the Shareholders Foundation.
Media Contact:
Michael Daniels
Shareholders Foundation, Inc.
3111 Camino Del Rio North
Suite 423
San Diego, CA 92108
Tel: +1-(858)-779-1554
E-Mail: mail@shareholdersfoundation.com
About Shareholders Foundation, Inc.
The Shareholders Foundation, Inc. is a professional portfolio monitoring and settlement claim filing service, and an investor advocacy group, which does research related to shareholder issues and informs investors of securities class actions, settlements, judgments, and other legal related news to the stock/financial market. Shareholders Foundation, Inc. is in contact with a large number of shareholders and offers help, support, and assistance for every shareholder. The Shareholders Foundation, Inc. is not a law firm. Referenced cases, investigations, and/or settlements are not filed/initiated/reached and/or are not related to Shareholders Foundation. The information is provided as a public service. It is not intended as legal advice and should not be relied upon.
Investors who purchased shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) have certain options and for certain investors are short and strict deadlines running. Deadline: August 17, 2020. NASDAQ: CEMI investors should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 - 1554.
In April 2020, Chembio Diagnostics, Inc’s Dual Path Platform (“DPP”) COVID-19 antibody test was among the first such tests to be granted Emergency Use Authorization (“EUA”) by the U.S. Food and Drug Administration (“FDA”).
The plaintiff alleggs that Defendants represented that the Company’s Dual Path Platform (“DPP”) COVID-19 serological point-of-care test for the detection of IgM and IgG antibodies aided in determining current or past exposure to the COVID-19 virus, that its test provides high sensitivity and specificity, and was 100% accurate.
Shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) reached as high as $14.90 per share on April 20, 2020.
The plaintiff claims that on May 11, 2020, Defendants took advantage of Chembio’s inflated stock price, closing a public offering of approximately 2.6 million shares of Chembio stock at $11.75 per share for gross proceeds of approximately $30.8 million.
However, then, on June 16, 2020, after the market closed, the U.S. Food and Drug Administration (“FDA”) issued a press release disclosing that it had revoked the Company’s Emergency Use Authorization (“EUA”) for the Company’s DPP COVID-19 Igm/IgG System “due to performance concerns with the accuracy of the test” and because “data submitted by Chembio as well as an independent evaluation of the Chembio test at NCI showed that this test generates a higher than expected rate of false results and higher than that reflected in the authorized labeling for the device.”
Shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) declined to as low as $3.51 per sahare on June 17, 2020.
The plaintiff claims that between April 1, 2020 and June 16, 2020 the Company failed to disclose that, the Company’s Dual Path Platform (“DPP”) COVID-19 test did not provide high-quality results and there were material performance concerns with the accuracy of the test such that it was not reasonable to believe that the test may be effective in detecting antibodies against SARS-CoV-2; and as a result, there was a material risk to public health from the false test results.
Those who purchased shares of Chembio Diagnostics, Inc. (NASDAQ: CEMI) have certain options and should contact the Shareholders Foundation.
Media Contact:
Michael Daniels
Shareholders Foundation, Inc.
3111 Camino Del Rio North
Suite 423
San Diego, CA 92108
Tel: +1-(858)-779-1554
E-Mail: mail@shareholdersfoundation.com
About Shareholders Foundation, Inc.
The Shareholders Foundation, Inc. is a professional portfolio monitoring and settlement claim filing service, and an investor advocacy group, which does research related to shareholder issues and informs investors of securities class actions, settlements, judgments, and other legal related news to the stock/financial market. Shareholders Foundation, Inc. is in contact with a large number of shareholders and offers help, support, and assistance for every shareholder. The Shareholders Foundation, Inc. is not a law firm. Referenced cases, investigations, and/or settlements are not filed/initiated/reached and/or are not related to Shareholders Foundation. The information is provided as a public service. It is not intended as legal advice and should not be relied upon.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
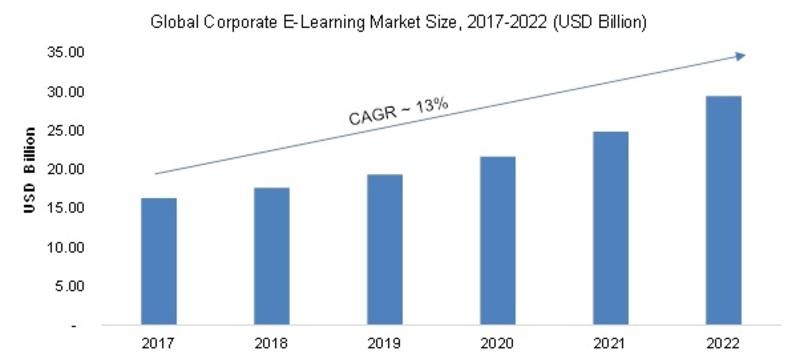
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
