Press release
MRI Safe Implantable Defibrillator Devices Market Forecast, Trend Analysis & Competition Tracking - Global Review 2017 to 2027
To study the market for MRI Safe Implantable Defibrillator Devices in a precise manner, Fact.MR has included a new research report. The report is titled “MRI Safe Implantable Defibrillator Devices Market Forecast, Trend Analysis & Competition Tracking - Global Review 2017 to 2027”, which enlightens the readers about the market share as well as growth rate during the period 2018-2027. The research study estimates that the MRI Safe Implantable Defibrillator Devices manufacturing sector would be rising with a positive CAGR during the same stated forecast period.
Request TOC of this Report- https://www.factmr.com/connectus/sample?flag=T&rep_id=566
Cardiac defibrillator is a battery powered pulse generator used to correct the abnormal heart rhythms by delivering an electric shock. It is very valuable device in preventing sudden deaths. For several years in the past, MRI was not allowed in patients having implanted devices such as cardiac defibrillator or pacemakers as it may alter pacing properties or even damage myocardium, thus might be raising concerns for clinicians. As, MRI is one of the most useful diagnostic test for patients with various medical problems including cardiology, neurology, orthopaedics etc., therefore the development of MRI safe implantable defibrillator devices helps in solving the problem of risks associated with MRI scans. Several research in the area proves that nearly two-third of patients implanted with a defibrillator needed an MRI diagnosis within four years of implantation, thereby generating the need of MRI safe implantable defibrillator devices.
The MRI safe implantable defibrillator devices are available as single chambered or dual chambered. These devices are either recognized as MR conditional or MR safe devices. They contain specific hardware and software components specifically tested and officially verified for use under magnetic resonance environment.
MRI Safe Implantable Defibrillator Devices Market: Drivers & Restraints
Several product advancements, rising geriatric population, increase in cardiac diseases and number of eligible population are some of the factors supporting the market growth of MRI safe implantable defibrillator devices. Furthermore, rising evidence about safety of these systems and effective marketing campaigns by various manufacturers supports the market growth of MRI safe implantable defibrillator devices. However, high product pricing and stringent regulatory issues for product approval lowers its market growth over the forecast period. Reimbursement issues, lack of skilled professionals in several countries are also limiting the market progress.
Browse Report- https://www.factmr.com/report/566/mri-safe-implantable-defibrillator-devices-market
MRI Safe Implantable Defibrillator Devices Market: Segmentation
Segmentation by Product Type:
Single Chamber Defibrillator
Dual Chamber Defibrillator
Segmentation by Modality:
MR Conditional Defibrillator
MR Safe Defibrillator
Segmentation by End User
Hospitals
Ambulatory Surgical Centers
MRI Safe Implantable Defibrillator Devices Market: Overview
There are very few companies involved in the manufacturing of devices with MRI compatibility. The products are launched in the market only few years back. Medtronic announced the CE Mark and European launch of first MRI safe implantable defibrillator devices to allow for full-body MRI Scans in April 2014. USFDA has also approved the first MRI compatible ICD manufactured by Medtronic recently in September 2015. Despite the launch of MRI safe implantable defibrillator devices, most of the population is still present with the non-MRI compatible devices. Companies in this direction are investing on research and development activities for advancement in these device types to improve therapy outcomes and manufacturing of low cost MRI safe implantable defibrillator devices. Top 3 companies in this market represent most of the market share globally for these MRI safe implantable defibrillator devices due to their strong presence, product approvals, brand strength and lack of competitors.
MRI Safe Implantable Defibrillator Devices Market: Region-wise Outlook
Geographically, North America leads the market for MRI Safe Implantable Defibrillator Devices services owing to the advanced healthcare facilities, high number of implants in the region as well as good reimbursement scenario. This is followed by the Western Europe countries due to easy approval of these devices by CE than by USFDA. Good adoption rate of MRI safe implantable defibrillator devices in the region promotes the growth. APEJ represents a significant growth opportunity for the MRI Safe Implantable Defibrillator devices over the forecast period owing to the rise in cardiac diseases, increase in healthcare spending and rising medical tourism. Japan also represents a substantial market share over the forecast period owing to the high number of geriatric population as well as available government support for medical facilities. Eastern Europe does not represent a significant market share due to less adoption rate of these devices. Latin America followed by Middle East & Africa represents the least market share as well as growth rate in the MRI Safe Implantable Defibrillator devices over the forecast period but represents a significant market opportunity for the market players because of the number of eligible population in these regions.
MRI Safe Implantable Defibrillator Devices Market Treatment Market: Key Players
Some of the players in the MRI Safe Implantable Defibrillator Devices market includes Medtronic, Boston Scientific Corporation, Abbott, Microport, Biotronik etc.
Pre-book Global MRI Safe Implantable Defibrillator Devices Market Forecast Report- https://www.factmr.com/checkout/566/S
About Fact.MR
Fact.MR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that’s why we publish multi-industry global, regional, and country-specific research reports.
Contact Us
Fact.MR
Suite 9884
27 Upper Pembroke Street,
Dublin 2, Ireland
Telephone: +353-1-6111-593
Email: sales@factmr.com/
Web: https://www.factmr.com/
Request TOC of this Report- https://www.factmr.com/connectus/sample?flag=T&rep_id=566
Cardiac defibrillator is a battery powered pulse generator used to correct the abnormal heart rhythms by delivering an electric shock. It is very valuable device in preventing sudden deaths. For several years in the past, MRI was not allowed in patients having implanted devices such as cardiac defibrillator or pacemakers as it may alter pacing properties or even damage myocardium, thus might be raising concerns for clinicians. As, MRI is one of the most useful diagnostic test for patients with various medical problems including cardiology, neurology, orthopaedics etc., therefore the development of MRI safe implantable defibrillator devices helps in solving the problem of risks associated with MRI scans. Several research in the area proves that nearly two-third of patients implanted with a defibrillator needed an MRI diagnosis within four years of implantation, thereby generating the need of MRI safe implantable defibrillator devices.
The MRI safe implantable defibrillator devices are available as single chambered or dual chambered. These devices are either recognized as MR conditional or MR safe devices. They contain specific hardware and software components specifically tested and officially verified for use under magnetic resonance environment.
MRI Safe Implantable Defibrillator Devices Market: Drivers & Restraints
Several product advancements, rising geriatric population, increase in cardiac diseases and number of eligible population are some of the factors supporting the market growth of MRI safe implantable defibrillator devices. Furthermore, rising evidence about safety of these systems and effective marketing campaigns by various manufacturers supports the market growth of MRI safe implantable defibrillator devices. However, high product pricing and stringent regulatory issues for product approval lowers its market growth over the forecast period. Reimbursement issues, lack of skilled professionals in several countries are also limiting the market progress.
Browse Report- https://www.factmr.com/report/566/mri-safe-implantable-defibrillator-devices-market
MRI Safe Implantable Defibrillator Devices Market: Segmentation
Segmentation by Product Type:
Single Chamber Defibrillator
Dual Chamber Defibrillator
Segmentation by Modality:
MR Conditional Defibrillator
MR Safe Defibrillator
Segmentation by End User
Hospitals
Ambulatory Surgical Centers
MRI Safe Implantable Defibrillator Devices Market: Overview
There are very few companies involved in the manufacturing of devices with MRI compatibility. The products are launched in the market only few years back. Medtronic announced the CE Mark and European launch of first MRI safe implantable defibrillator devices to allow for full-body MRI Scans in April 2014. USFDA has also approved the first MRI compatible ICD manufactured by Medtronic recently in September 2015. Despite the launch of MRI safe implantable defibrillator devices, most of the population is still present with the non-MRI compatible devices. Companies in this direction are investing on research and development activities for advancement in these device types to improve therapy outcomes and manufacturing of low cost MRI safe implantable defibrillator devices. Top 3 companies in this market represent most of the market share globally for these MRI safe implantable defibrillator devices due to their strong presence, product approvals, brand strength and lack of competitors.
MRI Safe Implantable Defibrillator Devices Market: Region-wise Outlook
Geographically, North America leads the market for MRI Safe Implantable Defibrillator Devices services owing to the advanced healthcare facilities, high number of implants in the region as well as good reimbursement scenario. This is followed by the Western Europe countries due to easy approval of these devices by CE than by USFDA. Good adoption rate of MRI safe implantable defibrillator devices in the region promotes the growth. APEJ represents a significant growth opportunity for the MRI Safe Implantable Defibrillator devices over the forecast period owing to the rise in cardiac diseases, increase in healthcare spending and rising medical tourism. Japan also represents a substantial market share over the forecast period owing to the high number of geriatric population as well as available government support for medical facilities. Eastern Europe does not represent a significant market share due to less adoption rate of these devices. Latin America followed by Middle East & Africa represents the least market share as well as growth rate in the MRI Safe Implantable Defibrillator devices over the forecast period but represents a significant market opportunity for the market players because of the number of eligible population in these regions.
MRI Safe Implantable Defibrillator Devices Market Treatment Market: Key Players
Some of the players in the MRI Safe Implantable Defibrillator Devices market includes Medtronic, Boston Scientific Corporation, Abbott, Microport, Biotronik etc.
Pre-book Global MRI Safe Implantable Defibrillator Devices Market Forecast Report- https://www.factmr.com/checkout/566/S
About Fact.MR
Fact.MR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that’s why we publish multi-industry global, regional, and country-specific research reports.
Contact Us
Fact.MR
Suite 9884
27 Upper Pembroke Street,
Dublin 2, Ireland
Telephone: +353-1-6111-593
Email: sales@factmr.com/
Web: https://www.factmr.com/
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
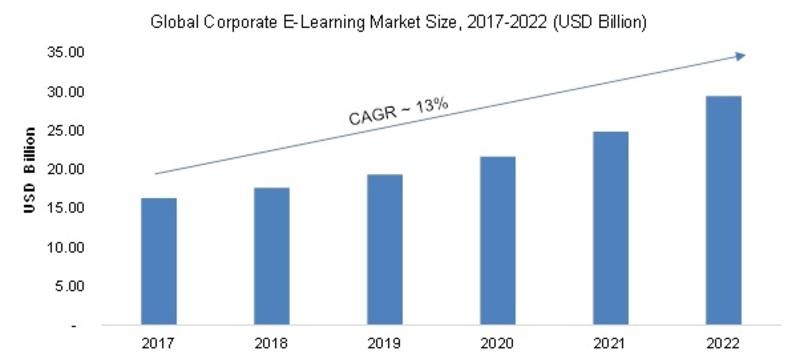
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
