Press release
Oncolytic Virus Cancer Therapy Pipeline Analysis: 120+ Companies are Working to Improve the Treatment Space | DelveInsight
DelveInsight's, "Oncolytic Virus Cancer Therapy Pipeline Insight, 2022," report provides comprehensive insights about 120+ companies and 125+ pipeline drugs in Oncolytic Virus Cancer Therapy pipeline landscape. It covers the pipeline drug profiles, including clinical and non-clinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Oncolytic Virus Cancer Therapy Pipeline Report
• DelveInsight's Oncolytic Virus Cancer Therapy Pipeline report depicts a robust space with 120+ active players working to develop 125+ pipeline therapies for Oncolytic Viruses Cancer treatment.
• Leading Oncolytic Virus Cancer Therapy companies such as Lokon Pharma, Merck, Wuhan Binhui Biotechnology, CG Oncology, Oncolys Biopharma, Replimune, Transgene, Sorrento Therapeutics, ORCA Therapeutics, TILT Biotherapeutics, BioEclipse Therapeutics, Oncolytics Biotech, Oncorus, DNAtrix, OncoMyx Therapeutics, Vyriad, Boehringer Ingelheim, IconOVir Bio, Imugene, Turnstone Biologics, Immvira Pharma, SillaJen Biotherapeutics, Advantagene, Nouscom, TOT Biopharm, Elicera Therapeutics, Takeda, Candel Therapeutics, Valo Therapeutics, Tessa Therapeutics, PsiOxus Therapeutics, KaliVir Immunotherapeutics, Oncos Therapeutics, Mustang Bio, Takara Bio, Vaxiion Therapeutics, Synthetic Biologics, Calidi Biotherapeutics, Genelux Corporation, Shanghai Sunway Biotech, BioInvent International AB, Guizhou Sinorda Biomedicine, EpicentRx, Hookipa Pharma Inc., Imvaq Therapeutics, Seneca Therapeutics, AmunBio, Orgenesis, Protheragen, Astellas Pharma, MedImmune LLC, Istari Oncology, Inc., Treovir LLC, and others are evaluating novel Oncolytic Virus Cancer Therapies to improve the treatment landscape.
• Key Oncolytic Virus Cancer Therapies in the pipeline in various stages of development include LOAd703, V937, OH2, CG0070, OBP-301, Vusolimogene oderparepvec, TG6002, Invir.IOTM based armed oncolytic immunotherapies, STI-1386, ORCA-010, TILT-123, CRX 100, Pelareorep, ONCR-177, ONCR-GBM, ONCR-021, ONCR-788, DNX-2401, DNX-2440, vMYX-hIL-12/Dec, Voyager-V1, MV-NIS, VCN 01, VCN 02, VCN 03, VCN 11, VSV-GP, CF33, RIVAL 01, MVR-T3011, Pexa-Vec, CAN-2409, CAN-3110, NOUS 209, NOUS PEV, TVP 211, ELC-100, ELC-201, Valo D102, TT 16, NG 641, NG 350A, NG 348, NG 347, VET2 L2, VET3 NK, VET1 S3, ONCOS-102, MB-108, HF10, VAX014, NNV-1, NNV 2, SNV 1, AAA1, Olvi-Vec, BT-001, SND005, AdAPT-001, SVV-001, Celyvir, RV-scFv-PDL1, OV FV 01, ASP9801, MEM 288, MEDI5395, PVSRIPO, G207, and others.
• The companies and academics are working to assess challenges and seek opportunities that could influence Oncolytic Virus Cancer Therapy R&D. The therapies under development are focused on novel approaches to treat/improve Oncolytic Virus Cancer Therapy.
Get an overview of the Oncolytic Virus Cancer Therapy Pipeline landscape @ https://www.delveinsight.com/sample-request/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Oncolytic Virus Cancer Therapy Overview
Oncolytic virotherapy is a treatment of using a virus that can replicate itself to kill cancer cells. There are many species of viruses, but not all of them can be designed to be an Oncolytic virus (OV). The typical features of these OVs must include being non-pathogenic, the ability to target and kill the cancer cells, and the capacity of being transformed to express tumor-killing factors through genetic engineering methods. Tumor selectivity could be concerned with the level of receptor-mediated cell entry, intracellular antiviral responses, or restriction factors that determine the susceptibility of the infected cell that leads to viral gene expression and replication.
Latest Breakthroughs and Developments in the Oncolytic Virus Cancer Therapy Pipeline
• In February 2022, Synthetic Biologics announced that VCN Biosciences, VCN-01 received Orphan Drug Designation for retinoblastoma from the US Food & Drug Administration (FDA).
• In March 2022, Synthetic Biologics announced that it has completed the acquisition of VCN Biosciences, S.L. (VCN) following the satisfaction of all closing conditions.
• In September 2021, CG Oncology announced a clinical trial collaboration to evaluate the safety and efficacy of CG0070, an oncolytic immunotherapy, in combination with OPDIVO (nivolumab), Bristol Myers Squibb's anti-PD-1 therapy, for the treatment of metastatic urothelial cancer in a Phase I/II clinical study.
• In March 2021, Orgenesis Inc. announced that it has entered the planned second phase of a collaboration with Hospital Infantil Universitario Niño Jesús in Madrid, Spain. The collaboration is focused on an exclusive license agreement to further develop and commercialize the hospital's proprietary Celyvir therapy for the treatment of solid tumors.
Oncolytic Virus Cancer Therapy Emerging Drugs
• Olvi-Vec: Genelux Corporation
• CAN-2409: Candel Therapeutics
• CG0070: CG Oncology
• Pelareorep: Oncolytics Biotech
• ParvOryx: Oryx GmbH
• SND005: Jiangsu Sinorda Biomedicine
• VCN-01: VCN Biosciences
Learn more about the novel and emerging Oncolytic Virus Cancer Therapy pipeline therapies @ https://www.delveinsight.com/sample-request/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Oncolytic Virus Cancer Therapy Pipeline Therapeutics Analysis
There are approx. 120+ key companies which are developing the therapies for Oncolytic Virus Cancer Therapy. The companies which have their Oncolytic Virus Cancer Therapy drug candidates in the most advanced stage, i.e. phase III include, Genelux Corporation.
DelveInsight's Oncolytic Virus Cancer Therapy Pipeline Report covers around 125+ products under different phases of clinical development like
• Late stage products (Phase III)
• Mid-stage products (Phase II)
• Early-stage product (Phase I) along with the details of
• Pre-clinical and Discovery stage candidates
• Discontinued & Inactive candidates
Dive deep into rich insights for oncolytic virus therapy, visit @ https://www.delveinsight.com/report-store/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Scope of the Oncolytic Virus Cancer Therapy Pipeline Report
• Coverage- Global
• Oncolytic Virus Cancer Therapy Companies- Lokon Pharma, Merck, Wuhan Binhui Biotechnology, CG Oncology, Amgen, Oncolys Biopharma, Replimune, Transgene, Sorrento Therapeutics, ORCA Therapeutics, TILT Biotherapeutics, BioEclipse Therapeutics, Oncolytics Biotech, Oncorus, DNAtrix, OncoMyx Therapeutics, Vyriad, Boehringer Ingelheim, IconOVir Bio, Imugene, Turnstone Biologics, Immvira Pharma, SillaJen Biotherapeutics, Advantagene, Nouscom, TOT Biopharm, Elicera Therapeutics, Takeda, Candel Therapeutics, Valo Therapeutics, Tessa Therapeutics, PsiOxus Therapeutics, KaliVir Immunotherapeutics, Oncos Therapeutics, Mustang Bio, Takara Bio, Vaxiion Therapeutics, Synthetic Biologics, Calidi Biotherapeutics, Genelux Corporation, Shanghai Sunway Biotech, BioInvent International AB, Guizhou Sinorda Biomedicine, EpicentRx, Hookipa Pharma Inc., Imvaq Therapeutics, Seneca Therapeutics, AmunBio, Orgenesis, Protheragen, Astellas Pharma, MedImmune LLC, Istari Oncology, Inc., Treovir LLC, and others.
• Oncolytic Virus Cancer Therapy Drugs- LOAd703, V937, OH2, CG0070, Talimogene laherparepvec, OBP-301, Vusolimogene oderparepvec, TG6002, Invir.IOTM based armed oncolytic immunotherapies, STI-1386, ORCA-010, TILT-123, CRX 100, Pelareorep, ONCR-177, ONCR-GBM, ONCR-021, ONCR-788, DNX-2401, DNX-2440, vMYX-hIL-12/Dec, Voyager-V1, MV-NIS, VCN 01, VCN 02, VCN 03, VCN 11, VSV-GP, CF33, RIVAL 01, MVR-T3011, Pexa-Vec, CAN-2409, CAN-3110, NOUS 209, NOUS PEV, TVP 211, ELC-100, ELC-201, Valo D102, TT 16, NG 641, NG 350A, NG 348, NG 347, VET2 L2, VET3 NK, VET1 S3, ONCOS-102, MB-108, HF10, VAX014, NNV-1, NNV 2, SNV 1, AAA1, Olvi-Vec, BT-001, SND005, AdAPT-001, SVV-001, Celyvir, RV-scFv-PDL1, OV FV 01, ASP9801, MEM 288, MEDI5395, PVSRIPO, G207, and others.
• Oncolytic Virus Cancer Therapy Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
• Oncolytic Virus Cancer Therapy Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
• Oncolytic Virus Cancer Therapy Therapeutics Assessment By Route of Administration: Intravenous, Intradermal, Intramuscular, Intravesicular, Intratumoral, Oral, Subcutaneous, Topical,
• Oncolytic Virus Cancer Therapy Therapeutics Assessment By Molecule Type: Vaccines, Small molecule, Polymers, Peptides, Monoclonal antibodies, Immunotherapy
Table of Content
1. Introduction
2. Executive Summary
3. Oncolytic Virus Cancer Therapy: Overview
4. Pipeline Therapeutics
5. Therapeutic Assessment
6. Oncolytic Virus Cancer Therapy- DelveInsight's Analytical Perspective
7. Late Stage Products (Phase III)
8. Olvi-Vec: Genelux Corporation
9. Mid Stage Products (Phase II)
10. SND005: Jiangsu Sinorda Biomedicine
11. Early Stage Products (Phase I/II)
12. AloCelyvir: Orgenesis
13. Early Stage Products (Phase I)
14. VCN-01: VCN Biosciences
15. Preclinical and Discovery Stage Products
16. SynOV 1.3: Beijing Syngentech
17. Inactive Products
18. Oncolytic Virus Cancer Therapy Key Companies
19. Oncolytic Virus Cancer Therapy Key Products
20. Oncolytic Virus Cancer Therapy- Unmet Needs
21. Oncolytic Virus Cancer Therapy- Market Drivers and Barriers
22. Oncolytic Virus Cancer Therapy- Future Perspectives and Conclusion
23. Oncolytic Virus Cancer Therapy Analyst Views
24. Oncolytic Virus Cancer Therapy Key Companies
25. Appendix
Find out more about viruses to kill cancer cells @ https://www.delveinsight.com/report-store/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Company Name: DelveInsight
Contact Person: Yash Bhardwaj
Email: ybhardwaj@delveinsight.com
Phone: +919650213330
Address: 304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for the healthcare domain and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Key Takeaways from the Oncolytic Virus Cancer Therapy Pipeline Report
• DelveInsight's Oncolytic Virus Cancer Therapy Pipeline report depicts a robust space with 120+ active players working to develop 125+ pipeline therapies for Oncolytic Viruses Cancer treatment.
• Leading Oncolytic Virus Cancer Therapy companies such as Lokon Pharma, Merck, Wuhan Binhui Biotechnology, CG Oncology, Oncolys Biopharma, Replimune, Transgene, Sorrento Therapeutics, ORCA Therapeutics, TILT Biotherapeutics, BioEclipse Therapeutics, Oncolytics Biotech, Oncorus, DNAtrix, OncoMyx Therapeutics, Vyriad, Boehringer Ingelheim, IconOVir Bio, Imugene, Turnstone Biologics, Immvira Pharma, SillaJen Biotherapeutics, Advantagene, Nouscom, TOT Biopharm, Elicera Therapeutics, Takeda, Candel Therapeutics, Valo Therapeutics, Tessa Therapeutics, PsiOxus Therapeutics, KaliVir Immunotherapeutics, Oncos Therapeutics, Mustang Bio, Takara Bio, Vaxiion Therapeutics, Synthetic Biologics, Calidi Biotherapeutics, Genelux Corporation, Shanghai Sunway Biotech, BioInvent International AB, Guizhou Sinorda Biomedicine, EpicentRx, Hookipa Pharma Inc., Imvaq Therapeutics, Seneca Therapeutics, AmunBio, Orgenesis, Protheragen, Astellas Pharma, MedImmune LLC, Istari Oncology, Inc., Treovir LLC, and others are evaluating novel Oncolytic Virus Cancer Therapies to improve the treatment landscape.
• Key Oncolytic Virus Cancer Therapies in the pipeline in various stages of development include LOAd703, V937, OH2, CG0070, OBP-301, Vusolimogene oderparepvec, TG6002, Invir.IOTM based armed oncolytic immunotherapies, STI-1386, ORCA-010, TILT-123, CRX 100, Pelareorep, ONCR-177, ONCR-GBM, ONCR-021, ONCR-788, DNX-2401, DNX-2440, vMYX-hIL-12/Dec, Voyager-V1, MV-NIS, VCN 01, VCN 02, VCN 03, VCN 11, VSV-GP, CF33, RIVAL 01, MVR-T3011, Pexa-Vec, CAN-2409, CAN-3110, NOUS 209, NOUS PEV, TVP 211, ELC-100, ELC-201, Valo D102, TT 16, NG 641, NG 350A, NG 348, NG 347, VET2 L2, VET3 NK, VET1 S3, ONCOS-102, MB-108, HF10, VAX014, NNV-1, NNV 2, SNV 1, AAA1, Olvi-Vec, BT-001, SND005, AdAPT-001, SVV-001, Celyvir, RV-scFv-PDL1, OV FV 01, ASP9801, MEM 288, MEDI5395, PVSRIPO, G207, and others.
• The companies and academics are working to assess challenges and seek opportunities that could influence Oncolytic Virus Cancer Therapy R&D. The therapies under development are focused on novel approaches to treat/improve Oncolytic Virus Cancer Therapy.
Get an overview of the Oncolytic Virus Cancer Therapy Pipeline landscape @ https://www.delveinsight.com/sample-request/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Oncolytic Virus Cancer Therapy Overview
Oncolytic virotherapy is a treatment of using a virus that can replicate itself to kill cancer cells. There are many species of viruses, but not all of them can be designed to be an Oncolytic virus (OV). The typical features of these OVs must include being non-pathogenic, the ability to target and kill the cancer cells, and the capacity of being transformed to express tumor-killing factors through genetic engineering methods. Tumor selectivity could be concerned with the level of receptor-mediated cell entry, intracellular antiviral responses, or restriction factors that determine the susceptibility of the infected cell that leads to viral gene expression and replication.
Latest Breakthroughs and Developments in the Oncolytic Virus Cancer Therapy Pipeline
• In February 2022, Synthetic Biologics announced that VCN Biosciences, VCN-01 received Orphan Drug Designation for retinoblastoma from the US Food & Drug Administration (FDA).
• In March 2022, Synthetic Biologics announced that it has completed the acquisition of VCN Biosciences, S.L. (VCN) following the satisfaction of all closing conditions.
• In September 2021, CG Oncology announced a clinical trial collaboration to evaluate the safety and efficacy of CG0070, an oncolytic immunotherapy, in combination with OPDIVO (nivolumab), Bristol Myers Squibb's anti-PD-1 therapy, for the treatment of metastatic urothelial cancer in a Phase I/II clinical study.
• In March 2021, Orgenesis Inc. announced that it has entered the planned second phase of a collaboration with Hospital Infantil Universitario Niño Jesús in Madrid, Spain. The collaboration is focused on an exclusive license agreement to further develop and commercialize the hospital's proprietary Celyvir therapy for the treatment of solid tumors.
Oncolytic Virus Cancer Therapy Emerging Drugs
• Olvi-Vec: Genelux Corporation
• CAN-2409: Candel Therapeutics
• CG0070: CG Oncology
• Pelareorep: Oncolytics Biotech
• ParvOryx: Oryx GmbH
• SND005: Jiangsu Sinorda Biomedicine
• VCN-01: VCN Biosciences
Learn more about the novel and emerging Oncolytic Virus Cancer Therapy pipeline therapies @ https://www.delveinsight.com/sample-request/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Oncolytic Virus Cancer Therapy Pipeline Therapeutics Analysis
There are approx. 120+ key companies which are developing the therapies for Oncolytic Virus Cancer Therapy. The companies which have their Oncolytic Virus Cancer Therapy drug candidates in the most advanced stage, i.e. phase III include, Genelux Corporation.
DelveInsight's Oncolytic Virus Cancer Therapy Pipeline Report covers around 125+ products under different phases of clinical development like
• Late stage products (Phase III)
• Mid-stage products (Phase II)
• Early-stage product (Phase I) along with the details of
• Pre-clinical and Discovery stage candidates
• Discontinued & Inactive candidates
Dive deep into rich insights for oncolytic virus therapy, visit @ https://www.delveinsight.com/report-store/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Scope of the Oncolytic Virus Cancer Therapy Pipeline Report
• Coverage- Global
• Oncolytic Virus Cancer Therapy Companies- Lokon Pharma, Merck, Wuhan Binhui Biotechnology, CG Oncology, Amgen, Oncolys Biopharma, Replimune, Transgene, Sorrento Therapeutics, ORCA Therapeutics, TILT Biotherapeutics, BioEclipse Therapeutics, Oncolytics Biotech, Oncorus, DNAtrix, OncoMyx Therapeutics, Vyriad, Boehringer Ingelheim, IconOVir Bio, Imugene, Turnstone Biologics, Immvira Pharma, SillaJen Biotherapeutics, Advantagene, Nouscom, TOT Biopharm, Elicera Therapeutics, Takeda, Candel Therapeutics, Valo Therapeutics, Tessa Therapeutics, PsiOxus Therapeutics, KaliVir Immunotherapeutics, Oncos Therapeutics, Mustang Bio, Takara Bio, Vaxiion Therapeutics, Synthetic Biologics, Calidi Biotherapeutics, Genelux Corporation, Shanghai Sunway Biotech, BioInvent International AB, Guizhou Sinorda Biomedicine, EpicentRx, Hookipa Pharma Inc., Imvaq Therapeutics, Seneca Therapeutics, AmunBio, Orgenesis, Protheragen, Astellas Pharma, MedImmune LLC, Istari Oncology, Inc., Treovir LLC, and others.
• Oncolytic Virus Cancer Therapy Drugs- LOAd703, V937, OH2, CG0070, Talimogene laherparepvec, OBP-301, Vusolimogene oderparepvec, TG6002, Invir.IOTM based armed oncolytic immunotherapies, STI-1386, ORCA-010, TILT-123, CRX 100, Pelareorep, ONCR-177, ONCR-GBM, ONCR-021, ONCR-788, DNX-2401, DNX-2440, vMYX-hIL-12/Dec, Voyager-V1, MV-NIS, VCN 01, VCN 02, VCN 03, VCN 11, VSV-GP, CF33, RIVAL 01, MVR-T3011, Pexa-Vec, CAN-2409, CAN-3110, NOUS 209, NOUS PEV, TVP 211, ELC-100, ELC-201, Valo D102, TT 16, NG 641, NG 350A, NG 348, NG 347, VET2 L2, VET3 NK, VET1 S3, ONCOS-102, MB-108, HF10, VAX014, NNV-1, NNV 2, SNV 1, AAA1, Olvi-Vec, BT-001, SND005, AdAPT-001, SVV-001, Celyvir, RV-scFv-PDL1, OV FV 01, ASP9801, MEM 288, MEDI5395, PVSRIPO, G207, and others.
• Oncolytic Virus Cancer Therapy Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
• Oncolytic Virus Cancer Therapy Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
• Oncolytic Virus Cancer Therapy Therapeutics Assessment By Route of Administration: Intravenous, Intradermal, Intramuscular, Intravesicular, Intratumoral, Oral, Subcutaneous, Topical,
• Oncolytic Virus Cancer Therapy Therapeutics Assessment By Molecule Type: Vaccines, Small molecule, Polymers, Peptides, Monoclonal antibodies, Immunotherapy
Table of Content
1. Introduction
2. Executive Summary
3. Oncolytic Virus Cancer Therapy: Overview
4. Pipeline Therapeutics
5. Therapeutic Assessment
6. Oncolytic Virus Cancer Therapy- DelveInsight's Analytical Perspective
7. Late Stage Products (Phase III)
8. Olvi-Vec: Genelux Corporation
9. Mid Stage Products (Phase II)
10. SND005: Jiangsu Sinorda Biomedicine
11. Early Stage Products (Phase I/II)
12. AloCelyvir: Orgenesis
13. Early Stage Products (Phase I)
14. VCN-01: VCN Biosciences
15. Preclinical and Discovery Stage Products
16. SynOV 1.3: Beijing Syngentech
17. Inactive Products
18. Oncolytic Virus Cancer Therapy Key Companies
19. Oncolytic Virus Cancer Therapy Key Products
20. Oncolytic Virus Cancer Therapy- Unmet Needs
21. Oncolytic Virus Cancer Therapy- Market Drivers and Barriers
22. Oncolytic Virus Cancer Therapy- Future Perspectives and Conclusion
23. Oncolytic Virus Cancer Therapy Analyst Views
24. Oncolytic Virus Cancer Therapy Key Companies
25. Appendix
Find out more about viruses to kill cancer cells @ https://www.delveinsight.com/report-store/oncolytic-virus-cancer-therapy-pipeline-insight-2021?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Company Name: DelveInsight
Contact Person: Yash Bhardwaj
Email: ybhardwaj@delveinsight.com
Phone: +919650213330
Address: 304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for the healthcare domain and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
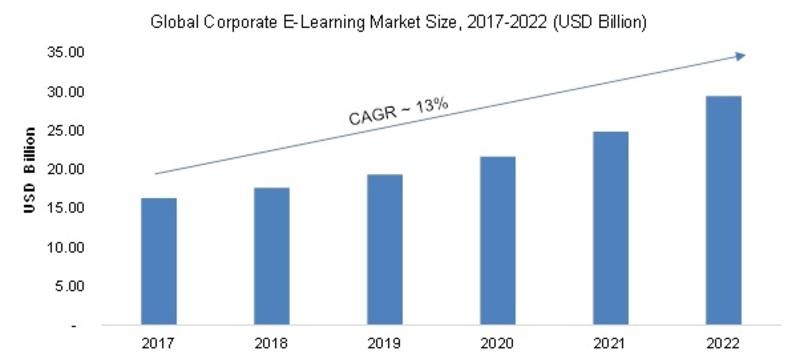
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
