Press release
One year left to comply with UK Medical Devices Regulations
London-based medical device regulatory consultancy Quality First International (QFI) reminds medical device manufacturers of the approaching end of the grace period for transitioning from compliance under the European Directives and Regulations for medical devices and IVDs
With the end of the transition period set for 30 June 2023, QFI encourages all manufacturers to apply to UK approved bodies as soon as possible to avoid discontinuation or failure to place products on the Great Britain market.
The UK Competent Authority (MHRA) will no longer accept medical device compliance under Council Directives 93/42/EEC, 90/385/EEC and 98/79/EC, nor Regulations (EU) 2017/745 / 2017/746 after 30 June 2023. Further, all manufacturers intending to place medical devices on the UK market must comply with the Medical Devices Regulations 2002 No. 618 (as amended) and apply a UK mark to their products.
"The new requirements apply to devices of all classes. We anticipate long conformity assessment processes with UK Approved Bodies", says Haroon Atchia, CEO and Technical Director at QFI. "Furthermore, the UK MHRA will only accept registration of devices from manufacturers where the manufacturer is based in the UK. If the manufacturer is based outside the UK, they must appoint a UK Responsible Person."
Quality First International
1 Cook's Road
Stratford
E15 2PW
Mariana Terentii
mariana@qualityfirstint.com
QFI is a London-based medical device consulting company specialising in solving its clients' regulatory, technical and compliance challenges to enable medical devices to enter the global marketplace. The company also acts as a UK Responsible Person for manufacturers located outside of the UK. QFI provides regulatory advice, quality assurance and clinical trial services, with particular emphasis on the European, US, Japanese, Australian, Canadian, Mexican and Chinese marketplaces. QFI's clients range from start-up companies to multinational organisations, primarily developing a broad range of cutting edge, minimally invasive and cardiovascular products and implants. QFI also specialises in various remediation and business excellence initiatives including solutions to regulatory enforcement, corporate integrity arrangements and expert witness services.
For further information please contact:
Tel: +44 (0)208 221 2361, Email: enquiries@qualityfirstint.com, Website: https://qualityfirstint.com
With the end of the transition period set for 30 June 2023, QFI encourages all manufacturers to apply to UK approved bodies as soon as possible to avoid discontinuation or failure to place products on the Great Britain market.
The UK Competent Authority (MHRA) will no longer accept medical device compliance under Council Directives 93/42/EEC, 90/385/EEC and 98/79/EC, nor Regulations (EU) 2017/745 / 2017/746 after 30 June 2023. Further, all manufacturers intending to place medical devices on the UK market must comply with the Medical Devices Regulations 2002 No. 618 (as amended) and apply a UK mark to their products.
"The new requirements apply to devices of all classes. We anticipate long conformity assessment processes with UK Approved Bodies", says Haroon Atchia, CEO and Technical Director at QFI. "Furthermore, the UK MHRA will only accept registration of devices from manufacturers where the manufacturer is based in the UK. If the manufacturer is based outside the UK, they must appoint a UK Responsible Person."
Quality First International
1 Cook's Road
Stratford
E15 2PW
Mariana Terentii
mariana@qualityfirstint.com
QFI is a London-based medical device consulting company specialising in solving its clients' regulatory, technical and compliance challenges to enable medical devices to enter the global marketplace. The company also acts as a UK Responsible Person for manufacturers located outside of the UK. QFI provides regulatory advice, quality assurance and clinical trial services, with particular emphasis on the European, US, Japanese, Australian, Canadian, Mexican and Chinese marketplaces. QFI's clients range from start-up companies to multinational organisations, primarily developing a broad range of cutting edge, minimally invasive and cardiovascular products and implants. QFI also specialises in various remediation and business excellence initiatives including solutions to regulatory enforcement, corporate integrity arrangements and expert witness services.
For further information please contact:
Tel: +44 (0)208 221 2361, Email: enquiries@qualityfirstint.com, Website: https://qualityfirstint.com
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
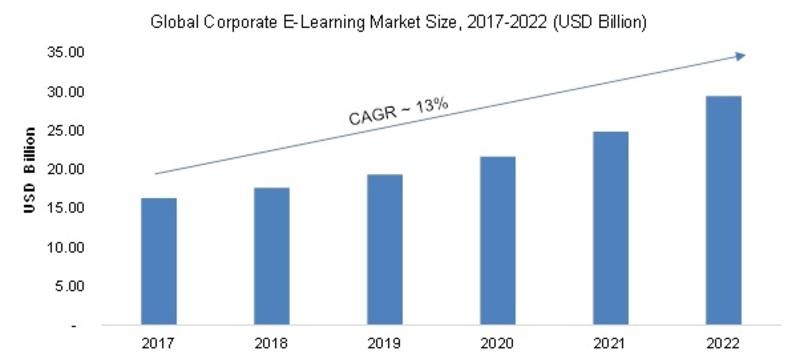
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...