Press release
Safety and Efficacy Profiles of Gamma Delta T Cell-Based Therapies
The safety and efficacy profiles of Gamma Delta T cell-based therapies are critical factors in their development and clinical application for cancer treatment. Understanding these profiles is essential for ensuring that gamma delta T cell therapies provide significant therapeutic benefits while minimizing potential risks to patients.
DOWNLOAD REPORT:
https://www.kuickresearch.com/report-gamma-delta-t-cell-therapy-market
Gamma delta T cells have demonstrated promising efficacy in preclinical studies and early-phase clinical trials. Their unique ability to recognize a wide range of tumor-associated antigens without the need for major histocompatibility complex (MHC) presentation allows them to target and eliminate various cancer types effectively. Clinical trials involving the adoptive transfer of ex vivo-expanded gamma delta T cells have shown significant anti-tumor activity in patients with advanced-stage cancers, including lung cancer, multiple myeloma, and colorectal cancer.
The efficacy of gamma delta T cell therapies can be attributed to several key mechanisms. Firstly, gamma delta T cells exhibit direct cytotoxicity against tumor cells by recognizing stress-induced ligands, such as MICA and MICB, on their surface. This direct killing mechanism is enhanced by the rapid response kinetics of gamma delta T cells, allowing for swift and potent anti-tumor activity. Additionally, gamma delta T cells can secrete pro-inflammatory cytokines like IFN-gamma and TNF-alpha, which enhance the overall immune response and contribute to the recruitment and activation of other immune cells.
Despite the promising efficacy, the safety profile of gamma delta T cell therapies must be carefully evaluated. Potential risks associated with gamma delta T cell therapies include off-target effects, cytokine release syndrome (CRS), and the potential for immune-related adverse events. Off-target effects occur when gamma delta T cells mistakenly target healthy cells, leading to unintended tissue damage. To mitigate this risk, researchers are developing strategies to enhance the specificity of gamma delta T cells, such as genetic engineering techniques that introduce chimeric antigen receptors (CARs) with high specificity for tumor antigens.
Cytokine release syndrome is another potential risk associated with gamma delta T cell therapies. CRS occurs when the infused T cells release large amounts of cytokines, leading to a systemic inflammatory response. While mild CRS can be managed with supportive care, severe CRS can be life-threatening. Monitoring patients closely and implementing strategies to manage CRS, such as the use of corticosteroids or anti-cytokine therapies, are essential for ensuring patient safety.
To ensure the safety and efficacy of gamma delta T cell-based therapies, rigorous preclinical testing and well-designed clinical trials are essential. Preclinical studies using animal models help to identify potential risks and optimize the therapeutic protocols. These studies provide valuable insights into the mechanisms of action, pharmacokinetics, and potential toxicities of gamma delta T cell therapies.
Clinical trials play a crucial role in evaluating the safety and efficacy of gamma delta T cell therapies in human patients. Phase I trials primarily focus on assessing the safety, tolerability, and optimal dosing of the therapies. These trials involve a small number of patients and closely monitor for adverse events. Phase II trials expand the patient population and assess the preliminary efficacy of the therapies. Finally, Phase III trials involve larger patient populations and aim to confirm the efficacy and safety of the therapies compared to standard treatments.
In conclusion, the safety and efficacy profiles of gamma delta T cell-based therapies are crucial factors in their development and clinical application for cancer treatment. While gamma delta T cells have shown promising efficacy in preclinical studies and early-phase clinical trials, ensuring patient safety is paramount. Rigorous preclinical testing, well-designed clinical trials, and strategies to mitigate potential risks, such as off-target effects and cytokine release syndrome, are essential for advancing gamma delta T cell therapies into mainstream oncology practice. As research and clinical applications continue to progress, gamma delta T cell-based therapies hold great promise for providing effective and safe treatment options for cancer patients.
KuicK Research
Delhi
India
Kuick Research is a market research and analytics company that provides targeted information for critical decisions at business, product and service levels. We are quick, predictive and known by the recommendations we have made in the past. Our result-oriented research methodology offers understanding of multiple issues in a short period of time and gives us the capability to keep you full with loads of practical ideas. By translating research answers into strategic insight and direction, we not only rate the success potential of your products and/or services, but also help you identify the opportunities for growth in new demographies and find ways to beat competition.
DOWNLOAD REPORT:
https://www.kuickresearch.com/report-gamma-delta-t-cell-therapy-market
Gamma delta T cells have demonstrated promising efficacy in preclinical studies and early-phase clinical trials. Their unique ability to recognize a wide range of tumor-associated antigens without the need for major histocompatibility complex (MHC) presentation allows them to target and eliminate various cancer types effectively. Clinical trials involving the adoptive transfer of ex vivo-expanded gamma delta T cells have shown significant anti-tumor activity in patients with advanced-stage cancers, including lung cancer, multiple myeloma, and colorectal cancer.
The efficacy of gamma delta T cell therapies can be attributed to several key mechanisms. Firstly, gamma delta T cells exhibit direct cytotoxicity against tumor cells by recognizing stress-induced ligands, such as MICA and MICB, on their surface. This direct killing mechanism is enhanced by the rapid response kinetics of gamma delta T cells, allowing for swift and potent anti-tumor activity. Additionally, gamma delta T cells can secrete pro-inflammatory cytokines like IFN-gamma and TNF-alpha, which enhance the overall immune response and contribute to the recruitment and activation of other immune cells.
Despite the promising efficacy, the safety profile of gamma delta T cell therapies must be carefully evaluated. Potential risks associated with gamma delta T cell therapies include off-target effects, cytokine release syndrome (CRS), and the potential for immune-related adverse events. Off-target effects occur when gamma delta T cells mistakenly target healthy cells, leading to unintended tissue damage. To mitigate this risk, researchers are developing strategies to enhance the specificity of gamma delta T cells, such as genetic engineering techniques that introduce chimeric antigen receptors (CARs) with high specificity for tumor antigens.
Cytokine release syndrome is another potential risk associated with gamma delta T cell therapies. CRS occurs when the infused T cells release large amounts of cytokines, leading to a systemic inflammatory response. While mild CRS can be managed with supportive care, severe CRS can be life-threatening. Monitoring patients closely and implementing strategies to manage CRS, such as the use of corticosteroids or anti-cytokine therapies, are essential for ensuring patient safety.
To ensure the safety and efficacy of gamma delta T cell-based therapies, rigorous preclinical testing and well-designed clinical trials are essential. Preclinical studies using animal models help to identify potential risks and optimize the therapeutic protocols. These studies provide valuable insights into the mechanisms of action, pharmacokinetics, and potential toxicities of gamma delta T cell therapies.
Clinical trials play a crucial role in evaluating the safety and efficacy of gamma delta T cell therapies in human patients. Phase I trials primarily focus on assessing the safety, tolerability, and optimal dosing of the therapies. These trials involve a small number of patients and closely monitor for adverse events. Phase II trials expand the patient population and assess the preliminary efficacy of the therapies. Finally, Phase III trials involve larger patient populations and aim to confirm the efficacy and safety of the therapies compared to standard treatments.
In conclusion, the safety and efficacy profiles of gamma delta T cell-based therapies are crucial factors in their development and clinical application for cancer treatment. While gamma delta T cells have shown promising efficacy in preclinical studies and early-phase clinical trials, ensuring patient safety is paramount. Rigorous preclinical testing, well-designed clinical trials, and strategies to mitigate potential risks, such as off-target effects and cytokine release syndrome, are essential for advancing gamma delta T cell therapies into mainstream oncology practice. As research and clinical applications continue to progress, gamma delta T cell-based therapies hold great promise for providing effective and safe treatment options for cancer patients.
KuicK Research
Delhi
India
Kuick Research is a market research and analytics company that provides targeted information for critical decisions at business, product and service levels. We are quick, predictive and known by the recommendations we have made in the past. Our result-oriented research methodology offers understanding of multiple issues in a short period of time and gives us the capability to keep you full with loads of practical ideas. By translating research answers into strategic insight and direction, we not only rate the success potential of your products and/or services, but also help you identify the opportunities for growth in new demographies and find ways to beat competition.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
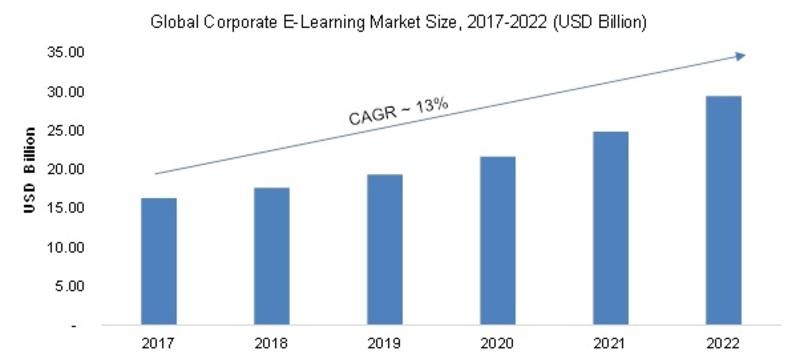
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...
