Press release
STEMart Introduces Medical Device Testing Services for Research Community
As a provider of integrated medical device CRO services dedicated to medical device and diagnostic clinical development, STEMart recently introduces medical device testing services for researchers all over the world. In accordance with ISO 10992 and ISO 18562 standards, FDA guidance, ASTM (American Society of Testing Materials) standards and other international guidelines, STEMart's experts with years of experience in a variety of testing services for Class, I, II and III medical devices now can provide assistance for clients to ensure that every aspect of medical device is properly inspected.
As medical devices are subject to risk, comprehensive medical device testing throughout the entire product life cycle is a critical step in the transformation process from an innovative design into a reliable and marketable product. STEMart now offers medical device testing services such as biocompatibility testing, microbiology & sterility, packaging solutions, facility & process validation, physical testing service, and preclinical medical device testing service for customers choice.
STEMart specializes in all kinds of fields including urology, neurology, dental, cardiovascular, ophthalmology, and imaging. It applies professional services throughout extensive experience conducting medical device clinical studies across multiple therapeutic areas to researchers' studies. Its medical expert services range from medical device design, prototyping, pilot production, testing, medical device certification services to the manufacturing processes.
"Combining expert medical product testing knowledge with the state-of-the-art laboratories and facilities, STEMart provides a one-stop, client-specific approach for testing of both active medical devices, non-active medical devices and In Vitro Diagnostic equipment. For instance, we can help manufacturers conduct a variety of In Vitro and In Vivo safety evaluation studies according to ISO 10993 and ISO 18562 standards on medical device to identify the presence of toxins or any other potentially harmful effects." said Tina Frederick, Executive Director of Marketing at STEMart.
"We're glad that we are able to bring comprehensive medical device testing services to scientific communities, and having this resource available within our facility will allow us to provide more professional services to our global clients. We can provide expert services to help medical device manufacturer validate terminal sterilization processes, and evaluate methods for cleaning and reprocessing reusable devices and instruments. We believe such newly offerings will advance our clients' important studies." said Brian N. Griffith, Product Portfolio Manager at STEMart.
If you want to know more details on medical device testing services or would like to consult with the experts at STEMart, please contact STEMart directly, or visit STEMart at https://www.ste-mart.com.
Staci Horme
Shirley, NY
About STEMart
STEMart is an industry-leading eCommerce platform with an expanded global footprint and has a broad portfolio of more than 10,000 products. It aims to provide best-in-class lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage towards the manufacturing process. STEMart is dedicated to making research and biotech production simpler and safer, and through that to accelerate access to better health for people everywhere.
As medical devices are subject to risk, comprehensive medical device testing throughout the entire product life cycle is a critical step in the transformation process from an innovative design into a reliable and marketable product. STEMart now offers medical device testing services such as biocompatibility testing, microbiology & sterility, packaging solutions, facility & process validation, physical testing service, and preclinical medical device testing service for customers choice.
STEMart specializes in all kinds of fields including urology, neurology, dental, cardiovascular, ophthalmology, and imaging. It applies professional services throughout extensive experience conducting medical device clinical studies across multiple therapeutic areas to researchers' studies. Its medical expert services range from medical device design, prototyping, pilot production, testing, medical device certification services to the manufacturing processes.
"Combining expert medical product testing knowledge with the state-of-the-art laboratories and facilities, STEMart provides a one-stop, client-specific approach for testing of both active medical devices, non-active medical devices and In Vitro Diagnostic equipment. For instance, we can help manufacturers conduct a variety of In Vitro and In Vivo safety evaluation studies according to ISO 10993 and ISO 18562 standards on medical device to identify the presence of toxins or any other potentially harmful effects." said Tina Frederick, Executive Director of Marketing at STEMart.
"We're glad that we are able to bring comprehensive medical device testing services to scientific communities, and having this resource available within our facility will allow us to provide more professional services to our global clients. We can provide expert services to help medical device manufacturer validate terminal sterilization processes, and evaluate methods for cleaning and reprocessing reusable devices and instruments. We believe such newly offerings will advance our clients' important studies." said Brian N. Griffith, Product Portfolio Manager at STEMart.
If you want to know more details on medical device testing services or would like to consult with the experts at STEMart, please contact STEMart directly, or visit STEMart at https://www.ste-mart.com.
Staci Horme
Shirley, NY
About STEMart
STEMart is an industry-leading eCommerce platform with an expanded global footprint and has a broad portfolio of more than 10,000 products. It aims to provide best-in-class lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage towards the manufacturing process. STEMart is dedicated to making research and biotech production simpler and safer, and through that to accelerate access to better health for people everywhere.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage
to this press release on woodPRI. woodPRI disclaims liability for any content contained in
this release.
Recommend
/newsMicroencapsulation Market Deep Analysis on Key Players - Dow Corning, Encapsys, Syngenta Crop Protection, Evonik Industries, 3M and Bayer
Market Study Report Adds Global Microencapsulation Market Size, Status and Forecast 2024 added to its database. The report provides key statistics on the current state of the industry and other analytical data to understand the market.
Extensive research is required for choosing the appropriate cor...

/newsGermany Airbag Market Size 2023: Global Share, Industry And Report Analysis By 2030 | Hyundai Mobis Co., Ltd. Key Safety Systems, Inc. Robert Bosch GmbH
Germany airbag market is expected to grow at a CAGR of around 6% during the forecast period. Germany Airbag Market research report refers to gathering and analyzing significant market data serve as best medium for various industry players to launch novel product or service. It is vital for key firms...
/newsSecurities Brokerages And Stock Exchanges Market Outlook 2021: Big Things are Happening
A new intelligence report released by HTF MI with title "Global Securities Brokerages And Stock Exchanges Market Survey & Outlook" is designed covering micro level of analysis by Insurers and key business segments, offerings and sales channels. The Global Securities Brokerages And Stock Exchange...

/newsRenewable Chemicals Market Emerging Trends and Competitive Landscape Forecast to 2028
The renewable chemicals market was valued at US$ 80,566.30 million in 2021 and is projected to reach US$ 1,76,750.76 million by 2028 it is expected to grow at a CAGR of 11.9% from 2021 to 2028. The research report focuses on the current market trends, opportunities, future potential of the market, a...
/newsHow Coronavirus is Impacting Cold Brew Coffee, Global Market Volume Analysis, Size, Share and Key Trends 2020-2026
"Market Latest Research Report 2020:
Los Angles United States, February 2020: The Cold Brew Coffee market has been garnering remarkable momentum in the recent years. The steadily escalating demand due to improving purchasing power is projected to bode well for the global market. QY Research's lates...
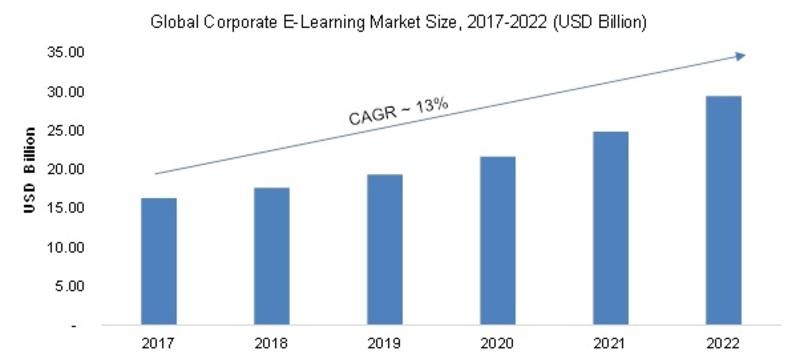
/newsCorporate E-Learning Market - Global Industry Size, Share, Key Players Analysis that are Infor, SkillSoft Corporation, Adrenna, CERTPOINT Systems and others with Regional Forecast to 2022
Overview:
E-Learning is used to enhance the learning procedures for newer job requirements and to make employees sound about the internal and external changes in the market and respective organizations. This method has created considerable differences in the ways of training and developing employee...